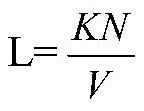Medical polymer sponge and method for removing initial contaminating bacteria thereof
A technology of macromolecules and polluting bacteria, applied in the medical field, can solve the problem of fever of patients, and achieve the effect of accelerating product aging, simple operation and easy industrialization.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] The sample numbers are 1#-1 and 1#-2 respectively.
[0030] The cleaning method in S1 is to wash according to the process of washing for 7 minutes and dehydrating for 6 minutes, and the process cycle is 9 times. Carry out secondary dehydration afterwards, the time of secondary dehydration is 8 minutes.
[0031] The drying condition in S2 is to dry for 3 hours at a temperature of 55°C.
Embodiment 2
[0033] The sample numbers are 2#-1 and 2#-2 respectively.
[0034] The cleaning method in S1 is to wash according to the process of washing for 5 minutes and dehydrating for 5 minutes, and the cycle is 7 times.
[0035] The drying condition in S2 is to dry at a temperature of 70° C. for 5 hours.
Embodiment 3
[0037] The sample numbers are 3#-1 and 3#-2 respectively.
[0038] The cleaning method in S1 is to wash according to the process of washing for 10 minutes and dehydrating for 8 minutes, and the cycle is 11 times. Carry out secondary dehydration afterwards, the time of secondary dehydration is 10 minutes.
[0039] The drying condition in S2 is to dry for 3 hours at a temperature of 50°C.
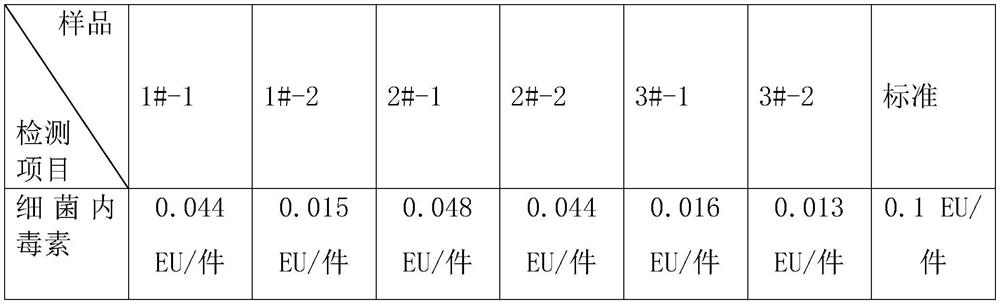
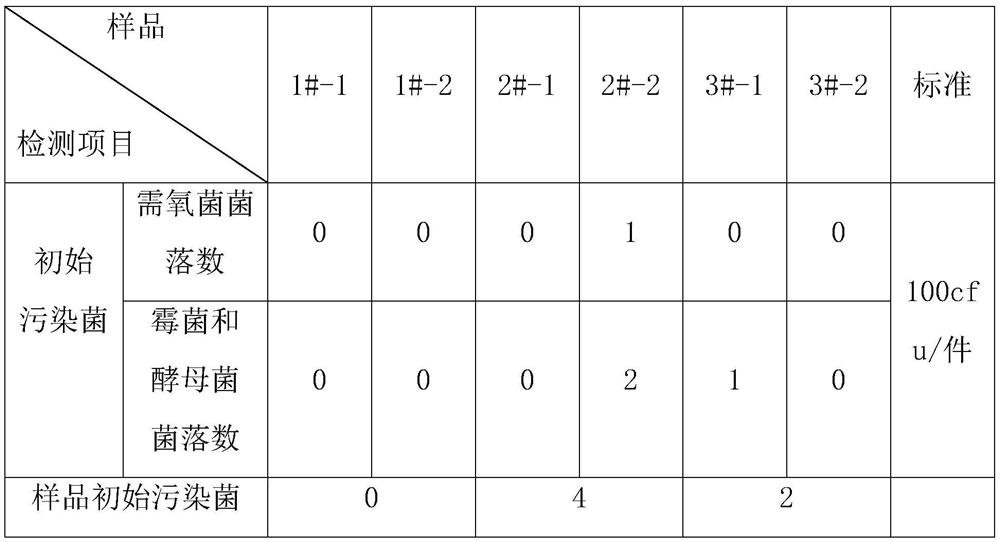
[0040] The specific test data of the medical polymer sponge after the above-mentioned three embodiments are as follows:
[0041] 1 bacterial endotoxin
[0042] Bacterial endotoxin standard formulation: According to GB / T 14233.2-2005 Medical infusion, blood transfusion, injection equipment inspection method Part 2: Biological test method 4.5.3 Recommended bacterial endotoxin limit for infusion, blood transfusion, and injection equipment in the preparation of test solution More than 20EU / piece.
[0043]
[0044] It should be noted that the calculation formula of the standard L is as foll...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com