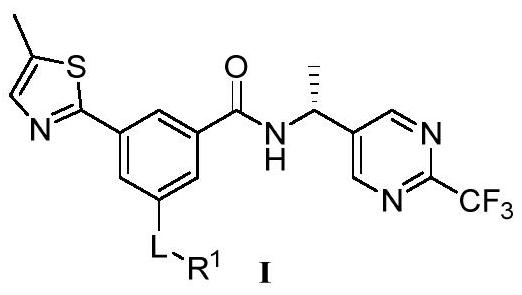
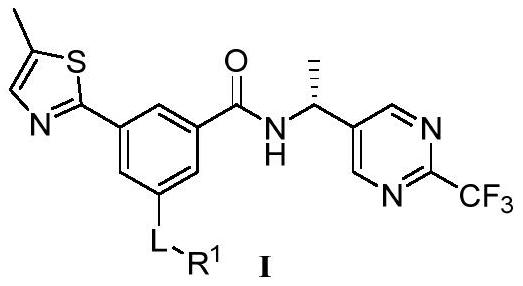
P2X3 inhibitors and uses thereof
A solvate, C3-C6 technology, applied in the field of medicinal chemistry, can solve the problems of unsuitability for long-term medication, unsuitable patients, adverse side effects, etc., and achieve excellent pharmacokinetic properties, good efficacy or druggability, and novel structure Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0101] Example 1: Preparation of target compound 1
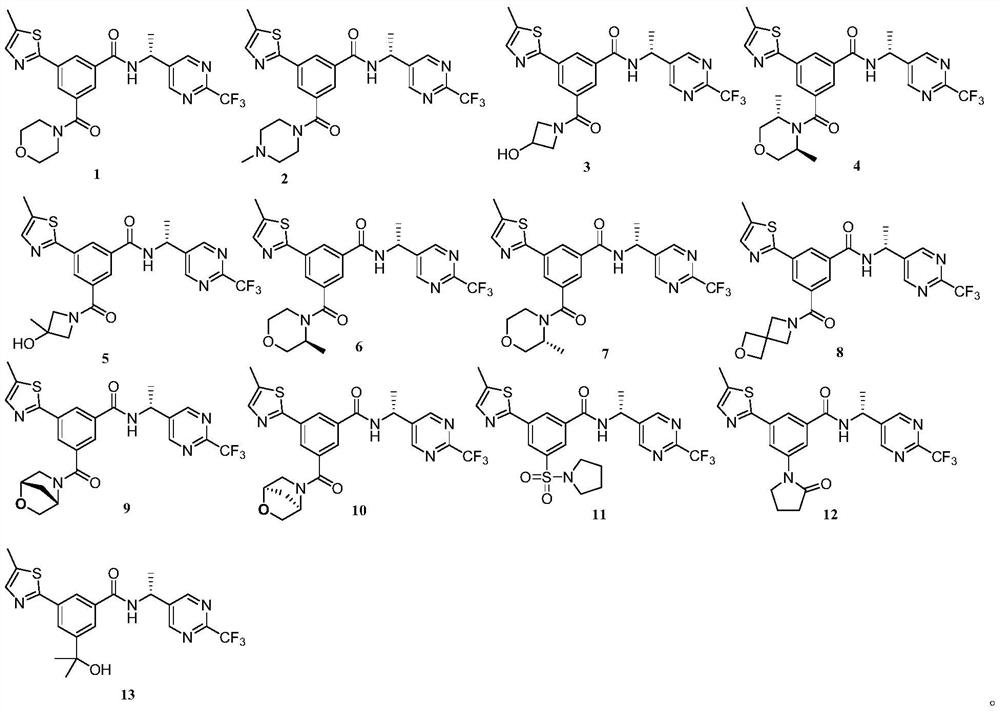
[0102] (R)-3-(5-Methylthiazol-2-yl)-5-(morpholine-4-carbonyl)-N-(1-(2-(trifluoromethyl)pyrimidin-5-yl)ethyl yl)benzamide (target compound 1)
[0103]
[0104] The synthetic route of target compound 1 is as follows:
[0105]
[0106] The first step: Synthesis of dimethyl 5-(4,4,5,5-tetramethyl-1,3,2-dioxaboran-2-yl)isophthalate (1B)
[0107]
[0108] 1,4-Dimethyl 5-bromoisophthalate (1A) (20 g, 73.2 mmol), pinacol Pd(dppf)Cl was added to the solution of dioxane (150 mL) 2 (2.68g, 3.66mmol), after replacing nitrogen three times, the reaction was carried out at 90°C for 16h. TLC showed that the reaction of the raw materials was complete, the system was cooled to room temperature and filtered, the filtrate was concentrated to dryness, and the residue was separated and purified by silica gel column to obtain the product 5-( Dimethyl 4,4,5,5-tetramethyl-1,3,2-dioxaboran-2-yl)isophthalate (1B) (13 g, 55.4% yield).
[...
Embodiment 2
[0130] Example 2: Preparation of target compound 2
[0131] (R)-3-(4-Methylpiperazine-1-carbonyl)-5-(5-methylthiazol-2-yl)-N-(1-(2-(trifluoromethyl)pyrimidine-5) -yl)ethyl)benzamide (target compound 2)
[0132]
[0133] The synthetic route of target compound 2 is as follows:
[0134]
[0135] The synthetic method refers to Example 1.
[0136] 1 H NMR (400MHz, DMSO-d 6 )δ9.29(d,1H),9.13(s,2H),8.41(t,1H),8.00-7.95(m,2H),7.69(d,1H),5.37-5.27(m,1H),3.66 (s,2H),3.42-3.37(m,1H),3.30-3.23(m,1H),2.53(d,3H),2.38(d,4H),2.23(s,3H),1.62(d,3H) ).
[0137] LC-MS, M / Z: 519.3 [M+H] + .
Embodiment 3
[0138] Example 3: Preparation of target compound 3
[0139] (R)-3-(3-Hydroxyazetidine-1-carbonyl)-5-(5-methylthiazol-2-yl)-N-(1-(2-(trifluoromethyl)pyrimidine) -5-yl)ethyl)benzamide (target compound 3)
[0140]
[0141] The synthetic route of target compound 3 is as follows:
[0142]
[0143] The synthetic method refers to Example 1.
[0144] 1 H NMR (400MHz, DMSO-d 6 )δ9.33(d,1H),9.14(s,2H),8.46(t,1H),8.19-8.15(m,2H),7.69(d,1H),5.81(d,1H),5.38-5.28 (m,1H),4.58-4.44(m,2H),4.37-4.25(m,1H),4.12-4.05(m,1H),3.88-3.80(d,1H),2.53(d,3H),1.63 (d,3H).
[0145] LC-MS, M / Z: 492.2 [M+H] + .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com