Bispecific neutralizing antibody for resisting novel coronavirus SARS-CoV-2 and application of bispecific neutralizing antibody
A technology of bispecific antibody and coronavirus, applied in the direction of antiviral agent, virus/bacteriophage, antiviral immunoglobulin, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0075] Example 1: Preparation of Nanobodies Recognizing SARS-CoV-2 RBD
[0076] Step 1: Rescue the phage surface-displayed nanobody library
[0077] The fully synthetic nanobody library is stored in the host bacteria in the form of phagemids. Before the panning process begins, the library is rescued to make it a phage-displayed antibody library. The specific method is as follows:
[0078] Take 1mL (OD 600 =100) antibody library Glycerol bacteria, inoculated into 5 bottles of 200mL 2TY-CARB medium (OD 600 =0.1), 37°C, 250rpm shake to OD 600 = about 0.5; add 1.6×10 12 PFU M13KO7, let stand at 37°C for 30 minutes, shake the bacteria at 37°C at 200rpm for 30 minutes; transfer the bacterial liquid to a centrifuge bottle, centrifuge at 2200g for 15 minutes, resuspend the bacteria with 400mL 2TY-CARB-KAN, 30°C, 250rpm Shake the bacteria for 14-16 hours; divide the bacteria solution into centrifuge bottles (200mL per bottle), centrifuge at 10000g, 4°C for 30 minutes, collect the ...
Embodiment 2
[0095] Embodiment 2: SARS-CoV-2 pseudotyped virus neutralizing activity assay
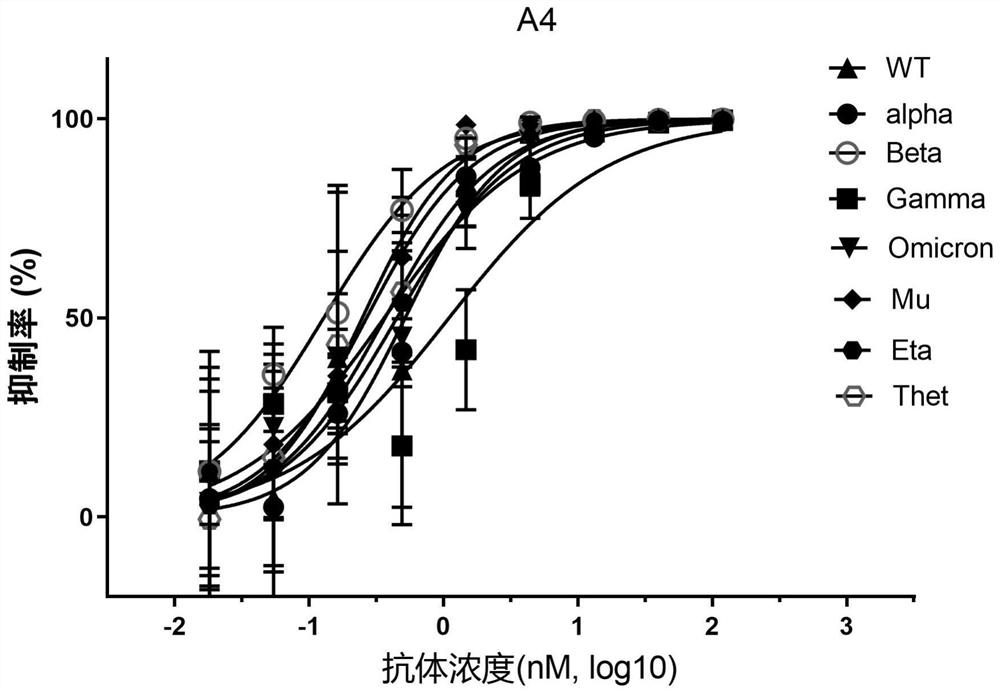
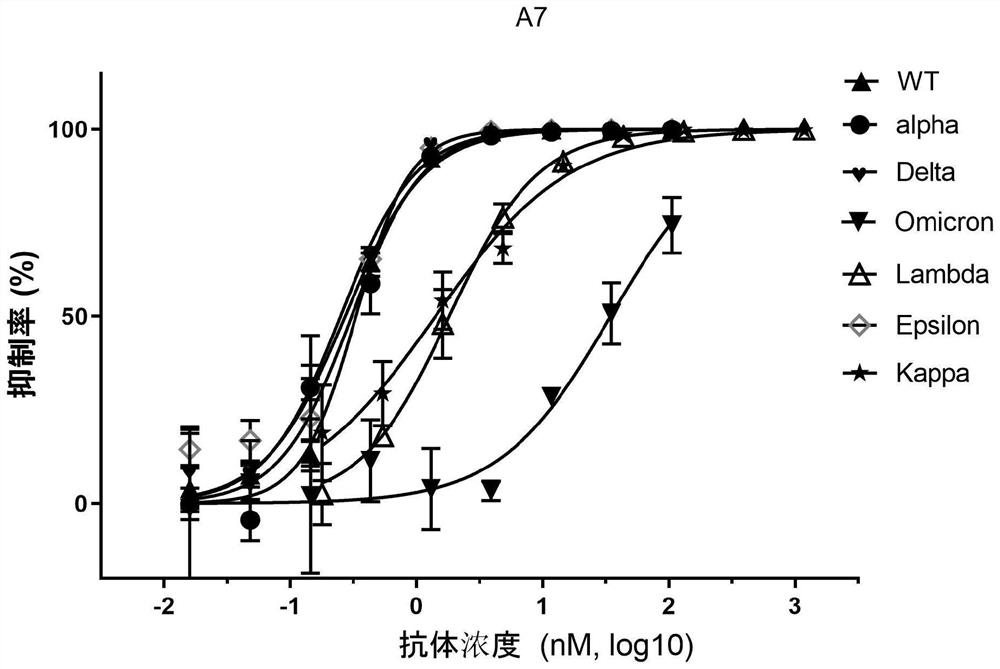
[0096] 10 4 293T stably transfected ACE2 cells (from the Cell Resource Center, Institute of Basic Medical Sciences, Chinese Academy of Medical Sciences) were inoculated in a 96-well plate, and SARS-CoV-2 and its mutant strain pseudotyped viruses were mixed with different dilutions of neutralizing nanobodies. , incubated at room temperature for 30 minutes, added to the cells, and after 6 hours of cultivation, the DMEM medium with 2% FBS was replaced, and the cultivation was continued for 48 hours. The pseudotyped virus of SARS-CoV-2 and its mutants contains a luciferase reporter gene, and the intracellular luciferase activity was detected using the luciferase reporter gene detection kit (Promega, catalog number: E4550), and the curve was fitted with GraphPad Prism 8 , calculate IC 50 value.
[0097] The experimental results of Nanobody A4 and Nanobody A7 inhibiting the invasion of SARS-CoV-2 pseudo...
Embodiment 3
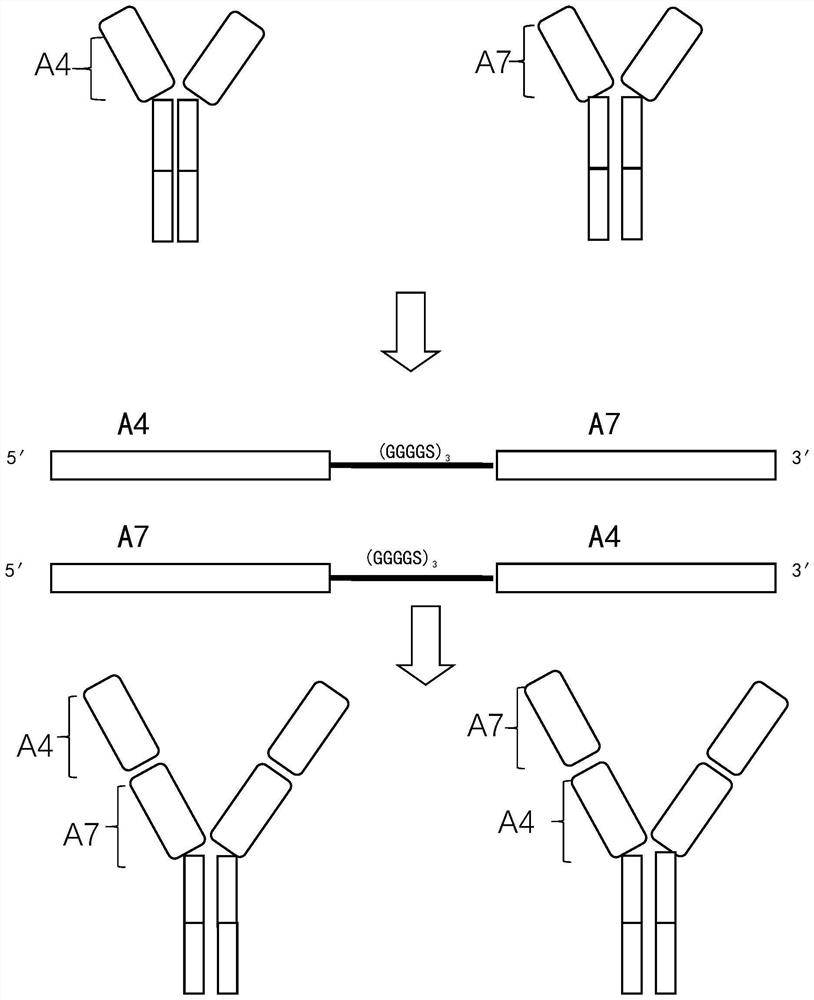
[0105] Example 3: Preparation of bispecific neutralizing antibodies
[0106] Step 1: Amplification and recombination of gene sequence
[0107] The recombinant plasmids of Nanobody A4 and Nanobody A7 obtained in Example 1 were used as templates for amplification respectively. Using the recombinant plasmid A4 as a template, use primers F and 4R to amplify to obtain gene fragment A4-1, and use primer 4F and primer R to amplify to obtain gene fragment A4-2; using recombinant plasmid A7 as a template, use primers F and 7R Amplify to obtain gene fragment A7-1, and use primer 7F and primer R to amplify to obtain gene fragment A7-2. The primers used are shown below.
[0108] F: 5'-caagtcaagctgctctgtg-3' (SEQ ID No. 12);
[0109] 4F:
[0110] 5'-agcggaggtgggggttcaggcggcggaggcagcgaagttcaattggttgaatc-3' (SEQ ID No. 13);
[0111] 4R: 5'-tgaacccccacctccgcttccgcctcctccagaagaaacagtaacttgag-3' (SEQ ID No. 14);
[0112] 7F:
[0113] 5'-agcggaggtgggggttcaggcggcggaggcagcgaagttcaactggttgaa...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com