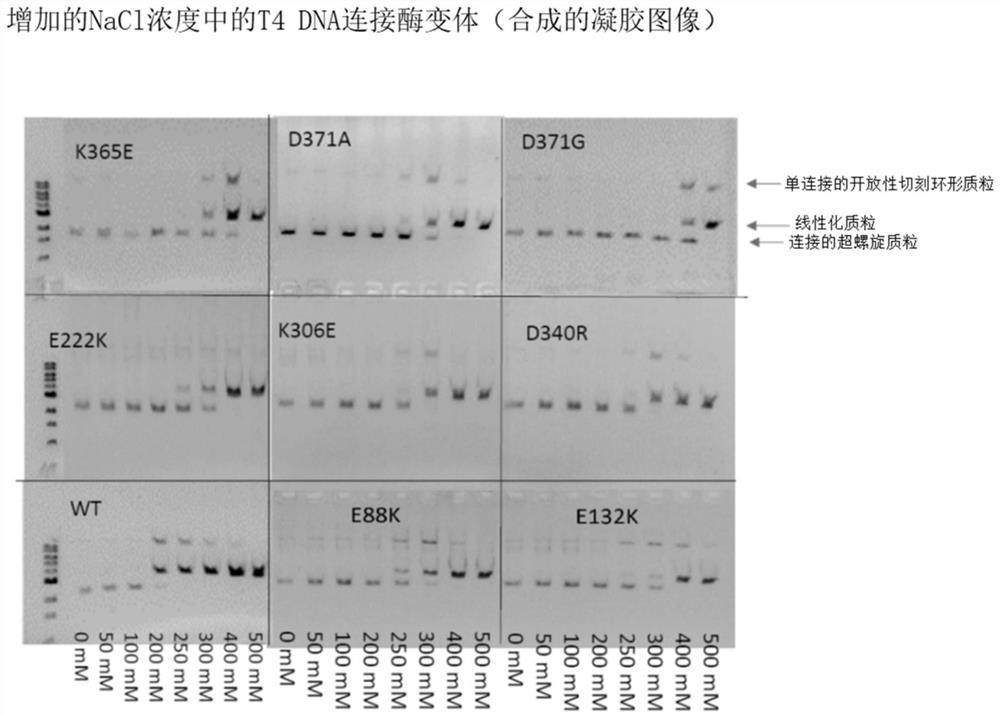
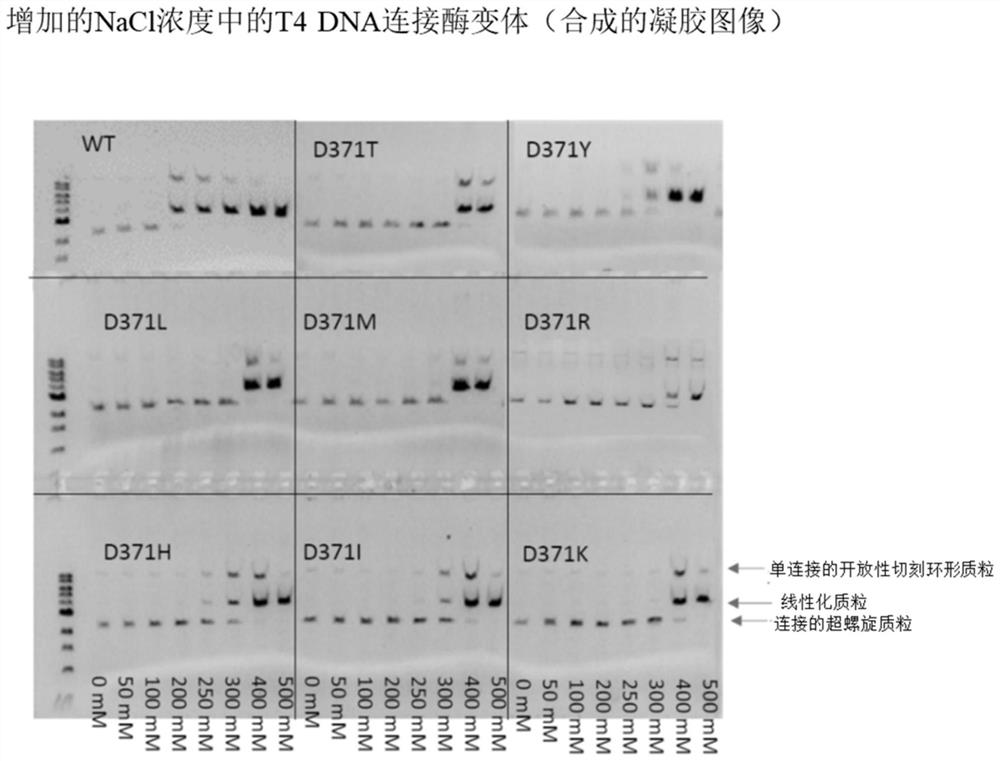
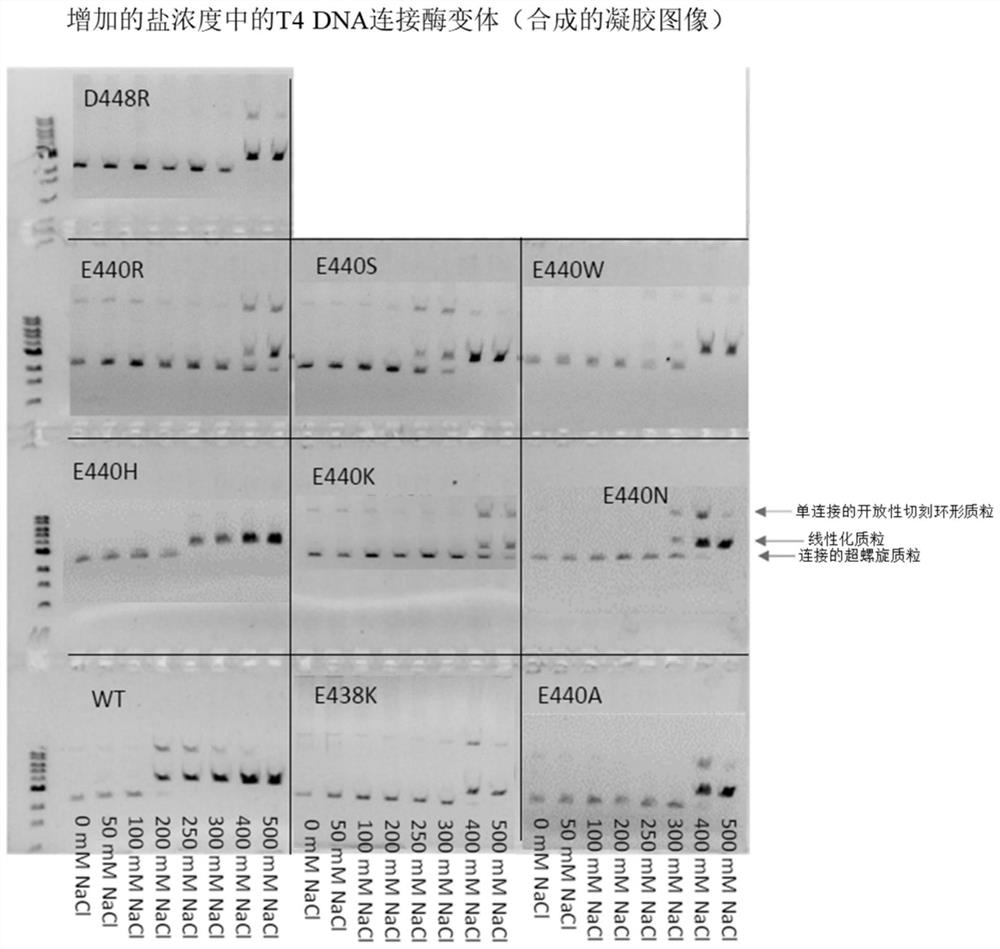
T4 DNA ligase variants with increased salt tolerance
A ligase, mutant technology, applied in the field of T4 DNA ligase variants, can solve the problems of sample loss, increase time and complexity, and achieve high activity effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0012] The term "biologically active fragment" refers to any fragment, derivative, homologue, or analog of a T4 DNA ligase mutant that has an in vivo or in vitro activity characteristic of a biomolecule; including, for example, ligase activity, or via a ligation repair band Mismatches present in nicked DNA. In some embodiments, the biologically active fragment, derivative, homologue or analog of the mutant T4 DNA ligase has any degree of biological activity of the mutant T4 DNA ligase in any in vivo or in vitro assay of interest .
[0013] In some embodiments, the biologically active fragment can optionally include any number of contiguous amino acid residues of a mutant T4 DNA ligase. The present invention also includes polynucleotides encoding any such biologically active fragments.
[0014] Biologically active fragments can result from post-transcriptional processing or translation of alternatively spliced RNAs, or alternatively can be produced by engineering, batch syn...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com