Amino-acid ester derivative and application thereof
A technology of alkylation and drugs, which is applied in the direction of drug combination, organic chemistry, antineoplastic drugs, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
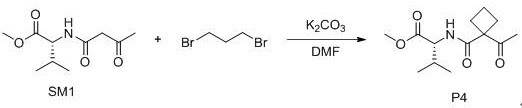
[0023] Example 1: Synthesis of Compound P1
[0024]
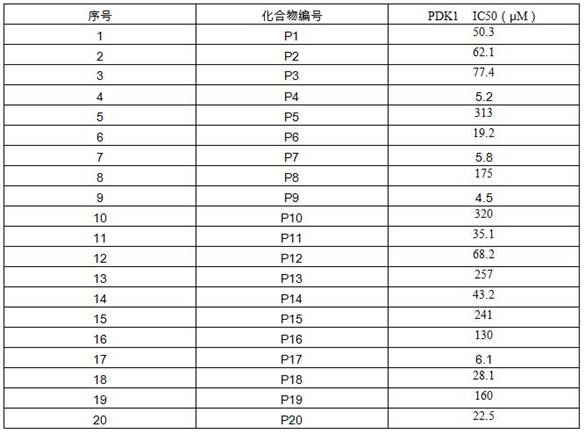
[0025] SM1 (500mg, 2.15mmol), potassium carbonate (653mg, 4.73mmol), 30ml DMF were added to a reaction flask with a U-shaped drying tube, stirred at room temperature for 1h, and then iodomethane (1.5g, 10.75mmol) was added, TLC detected that the reaction was complete, 20 ml of water and 20 ml of ethyl acetate were added, and the liquids were separated by stirring. The ethyl acetate phase was retained, washed with saturated NaCl aqueous solution, and anhydrous Na 2 SO 4 Drying, filtration, rotary evaporation, and silica gel column chromatography of the crude product (ethyl acetate:petroleum ether=10:1) yielded 220 mg of yellow oil, with a yield of 42%. ESI-MS: 244.15 [M+H] + .
[0026] 1H NMR (400 MHz, DMSO-d 6 ) δ 7.89 (d, J = 7.7 Hz, 1H), 4.11 (t, J =7.7 Hz, 1H), 3.63 (s, 3H), 2.08 (s, 4H), 1.27 (d, J = 5.0 Hz, 6H) ), 0.88 (dd,J = 8.1, 6.9 Hz, 6H).
Embodiment 2
[0027] Example 2: Synthesis of Compound P2
[0028]
[0029] Add SM1 (500mg, 2.15mmol), potassium carbonate (653mg, 4.73mmol), 30ml DMF to a reaction flask with a U-shaped drying tube, stir at room temperature for 1h, then add iodomethane (305mg, 3.15mmol), TLC Detection, the reaction is complete, add 20 ml of water and 20 ml of ethyl acetate, stir and separate the liquid, retain the ethyl acetate phase, wash with saturated NaCl aqueous solution, and anhydrous Na 2 SO 4 Drying, filtration, rotary evaporation, and the crude product was subjected to silica gel column chromatography (ethyl acetate: petroleum ether=10:1) to obtain 276 mg of yellow oil, yield 56%. ESI-MS: 230.28 [M+H] + .
[0030] 1H NMR (400 MHz, DMSO-d 6 ) 8.56 (dd, J = 20.0, 7.9 Hz, 1H), 4.20 (ddd, J = 15.7, 8.1, 6.2 Hz, 1H), 3.66 (d, J = 5.2 Hz, 3H), 3.33 – 3.28 (m, 1H), 2.10 (dd, J = 10.5, 4.1 Hz, 4H), 1.10 (dd, J = 6.9, 3.9 Hz, 3H), 0.97 –0.82 (m, 6H).
Embodiment 3
[0031] Example 3: Synthesis of Compound P3
[0032]
[0033] SM1 (500mg, 2.32mmol), potassium carbonate (652mg, 4.73mmol), 30ml DMF were added to a reaction flask with a U-shaped drying tube, stirred at room temperature for 1h, and then 1,2-dibromoethane (525mg) was added. , 2.79 mmol), TLC detection, the reaction is complete, add 20 ml of water and 20 ml of ethyl acetate, stir and separate the liquids, retain the ethyl acetate phase, wash with saturated NaCl aqueous solution, anhydrous Na 2 SO 4 Drying, filtration, rotary evaporation, the crude product was subjected to silica gel column chromatography (ethyl acetate: petroleum ether = 10:1) to obtain 320 mg of yellow oil, yield 57%. ESI-MS: 242.29 [M+H] + .
[0034] 1H NMR (400 MHz, CDCl 3 ) δ 9.36 (d, J = 6.8 Hz, 1H), 4.49 (dd, J =8.0, 5.0 Hz, 1H), 3.74(s, 3H), 2.23 (m, 1H), 2.01 (s, 3H), 1.90 – 1.82 (m, 2H), 1.59 – 1.51 (m, 2H), 0.99 (dd, J = 6.9, 1.9 Hz, 6H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com