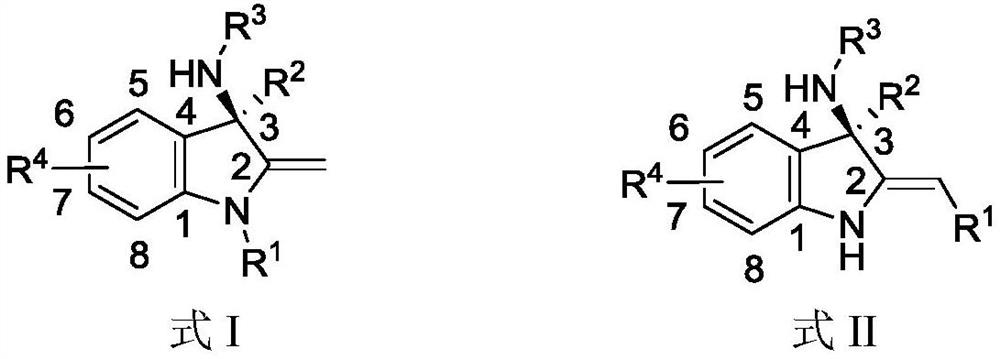
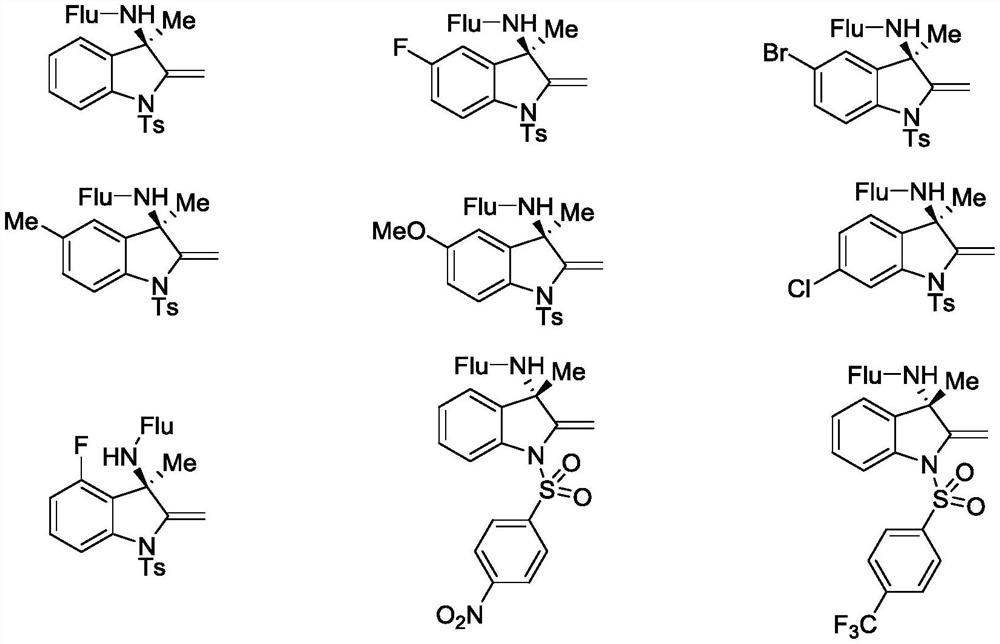
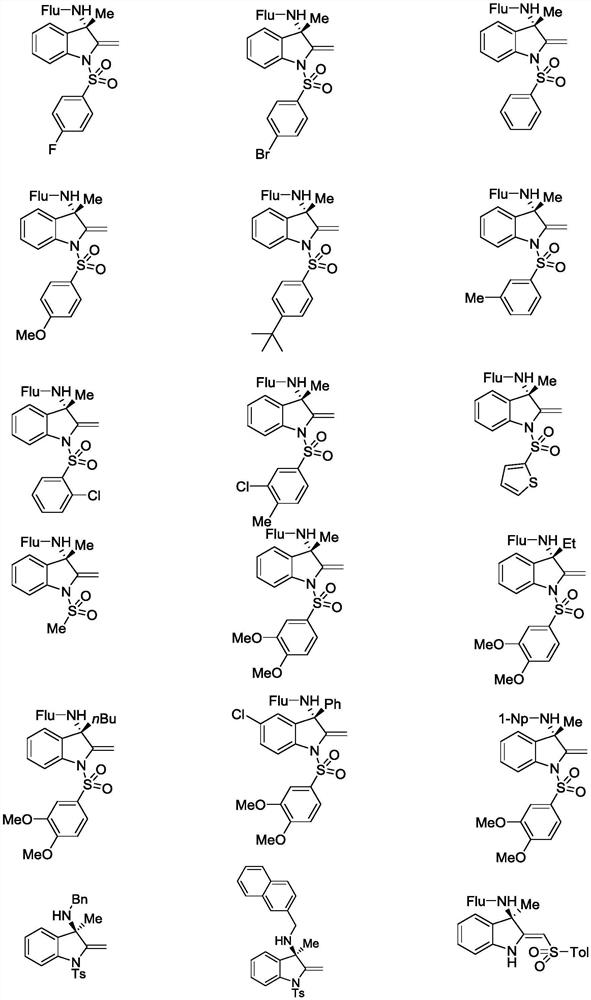
Chiral amino indoline derivative as well as preparation method and application thereof
A technology of aminoindoline and derivatives, which is applied in the direction of drug combination, pharmaceutical formula, organic active ingredients, etc., can solve the problems of expensive and harsh reaction conditions, and achieve simple preparation steps, mild conditions, and high enantioselectivity Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0047] Preparation of compound I-1:
[0048]
[0049] As shown in Reaction A, at room temperature, copper sulfate (0.01 mmol, 10 mol%) was mixed with ligand L 1 (0.012 mmol, 12 mol%) was dissolved in 3 mL of methanol and stirred under argon for 15 minutes. Then add base B 3 (0.2 mmol, 2.0 equiv.), stirring was continued for 15 minutes, followed by the addition of ethynyl benzoxazinone III (0.1 mmol, 1.0 equiv, where R 1 is -Ts, where R 2 for -ch3 , R 4 For H) and amine source IV (0.2mmol, 2.0equiv, R 3 For fluorenyl-Flu), the reaction mixture was continued to react at 30 ° C until the reaction was completed by TLC detection, using column chromatography gradient elution, with petroleum ether-ethyl acetate (V 石油醚 / V 乙酸乙酯 From 15:1 to 8:1) as a gradient elution solvent, the target product of formula I-1 was obtained in a yield of 86%.
[0050] Reaction formula A:
[0051]
[0052] Structural characterization data of product I-1: 1 H NMR (400MHz, CDCl 3 )δ(ppm)=8.1...
Embodiment 2
[0056] Preparation of compound I-2
[0057]
[0058] The reaction formula is shown in formula A. At room temperature, copper sulfate (0.01 mmol, 10 mol%) and ligand V (0.012 mmol, 12 mol%) were dissolved in 3 mL of methanol, and stirred for 15 minutes under argon protection. Then base B3 (0.2 mmol, 2.0 equiv.) was added, stirring was continued for 15 minutes, followed by addition of ethynyl benzoxazinone II (0.1 mmol, 1.0 equiv, where R 1 for -ts,r 2 for -ch 3 , R 4 6-F) and amine source IV (0.2mmol, 2.0equiv, R 3 For fluorenyl-Flu), the reaction mixture was continued to react at 30 ° C until the reaction was completed by TLC detection, using column chromatography gradient elution, with petroleum ether-ethyl acetate (V 石油醚 / V 乙酸乙酯 From 15:1 to 8:1) as a gradient elution solvent, the target product of formula I-2 was obtained in 88% yield.
[0059] Structural characterization data for product I-2: 1 H NMR (400MHz, CDCl 3 )δ(ppm)=8.05(dd,J=9.1,4.4Hz,1H),7.57-7.47(m,4H...
Embodiment 3
[0062] Preparation of compound I-3
[0063]
[0064] Copper sulfate (0.01 mmol, 10 mol %) and ligand V (0.012 mmol, 12 mol %) were dissolved in 3 mL of methanol at room temperature and stirred under argon for 15 minutes. Then base B3 (0.2 mmol, 2.0 equiv.) was added, stirring was continued for 15 minutes, followed by addition of ethynyl benzoxazinone II (0.1 mmol, 1.0 equiv, where R 1 For p-toluenesulfonyl-Ts, R 2 for -ch 3 , R 4 6-Br) and amine source IV (0.2mmol, 2.0equiv, R 3 For fluorenyl-Flu), the reaction mixture was continued to react at 30 ° C until the reaction was completed by TLC detection, using column chromatography gradient elution, with petroleum ether-ethyl acetate (V 石油醚 / V 乙酸乙酯 From 15:1 to 8:1) as a gradient elution solvent, the target product of formula I-3 was obtained in 83% yield.
[0065] Product structure characterization data: 1 H NMR (400MHz, CDCl 3 )δ(ppm)=7.97(d,J=8.7Hz,1H),7.60-7.42(m,6H),7.34-7.13(m,5H),7.02(m,J=7.5,1.2Hz,1H), 6.66(d,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com