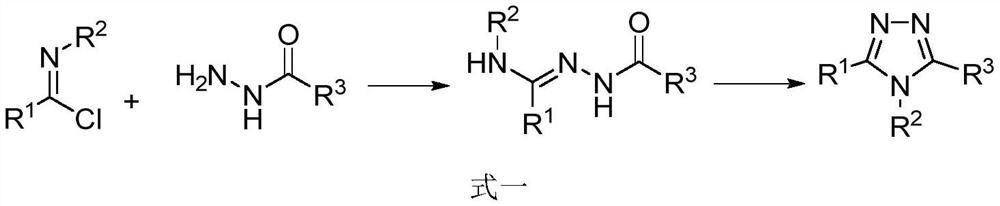
Preparation of 1, 2, 4-triazole
A technology of reaction and phenyl, applied in the field of preparation of 1,2,4-triazole, can solve the problems of unfavorable experimental operation, industrial production, harsh reaction conditions, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
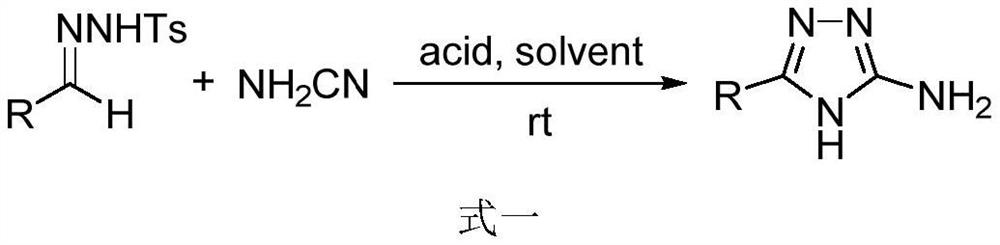
[0020] Embodiment 1: the preparation method of 3-amino-1,2,4-triazole:
[0021] Under argon, benzenesulfonyl hydrazone (0.2 mmol) was dissolved in 1,2-dichloroethane (3 mL), and NH was added. 2 CN (0.4 mmol), BF 3 ·OEt 2 (0.3 mmol), react at room temperature until the benzenesulfonyl hydrazone completely disappears (5-24 hours), the product is extracted with ethyl acetate (200 mL), and the organic phase is washed with saturated Na 2 CO 3 Solution wash, saline wash, anhydrous MgSO 4 After drying, the solvent was evaporated under reduced pressure, and 3-amino-1,2,4-triazole was obtained by column chromatography.
[0022] The spectral data are as follows:
[0023]
[0024] 14.9mg, 93% yield. 1 H NMR (400MHz, DMSO-d 6 )δ=12.05(brs,1H),7.91-7.88(m,2H),7.42-7.34(m,3H),6.07(brs,2H); 13 C NMR (100MHz, DMSO-d 6 )δ=158.4, 157.3, 132.3, 128.4, 128.1, 125.3ppm.
[0025]
[0026] 30.3mg, 87% yield. 1 H NMR (400MHz, DMSO-d 6 )δ=11.98(brs,1H),7.78(d,J=8.4Hz,2H),7.20(d,J=7.2...
Embodiment 2
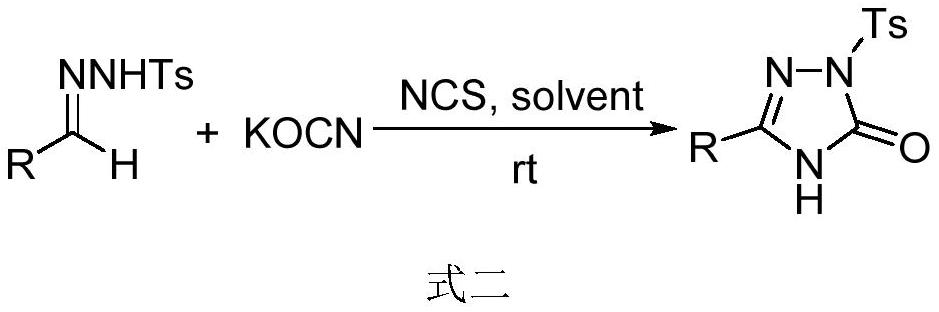
[0055] Embodiment 2: The preparation method of 2-toluenesulfonyl-1,2,4-triazol-3-one:
[0056] Under argon protection, dissolve benzenesulfonylhydrazone (0.2mmol) in acetonitrile (3mL), add KOCN (0.4mmol), NCS (0.22mmol), and react at room temperature until benzenesulfonylhydrazone disappears completely (5-18 hours) , the product was extracted with ethyl acetate (200 mL), and the organic phase was washed with saturated Na 2 CO 3 Solution wash, saline wash, anhydrous MgSO 4 After drying, the solvent was evaporated under reduced pressure, and 2-toluenesulfonyl-1,2,4-triazol-3-one was isolated by column chromatography.
[0057] The spectral data are as follows:
[0058]
[0059] 43.5 mg, 69% yield. 1 H NMR (400 MHz, DMSO-d 6 )δ=12.85(brs,1H),7.93-7.81(m,4H),7.57-7.49(m,5H),2.40(s,3H); 13 C NMR (100 MHz, DMSO-d 6 )δ=152.3, 147.8, 145.9, 133.9, 131.6, 130.3, 129.1, 127.5, 125.8, 125.1, 21.1 ppm; HRMS(ESI):Calcd forC 15 H 14 N 3 O 3 S[M+H] + :316.0750,found:316.0753. ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com