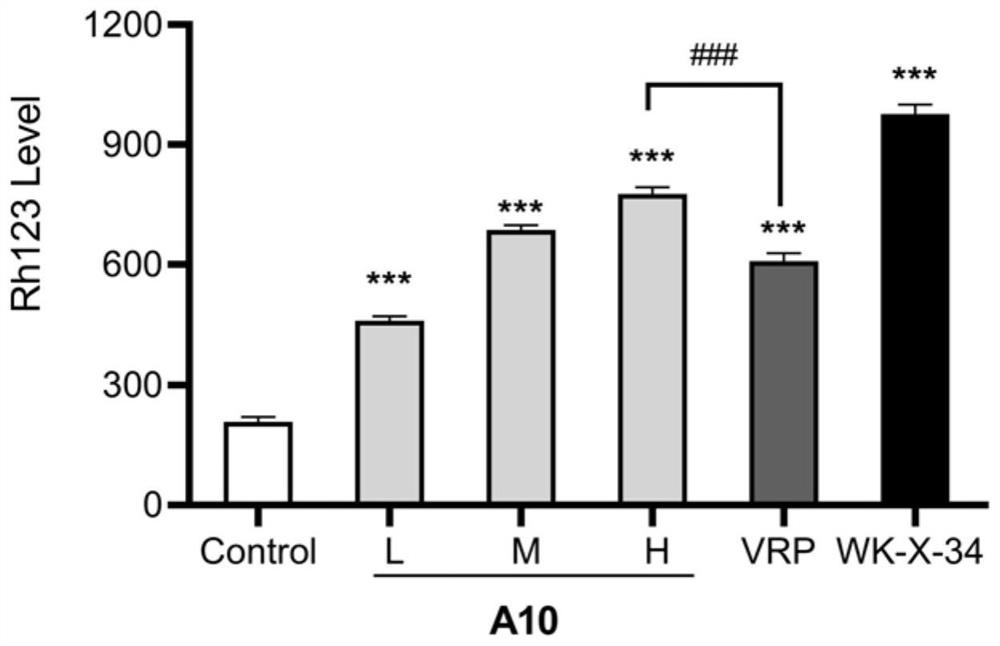
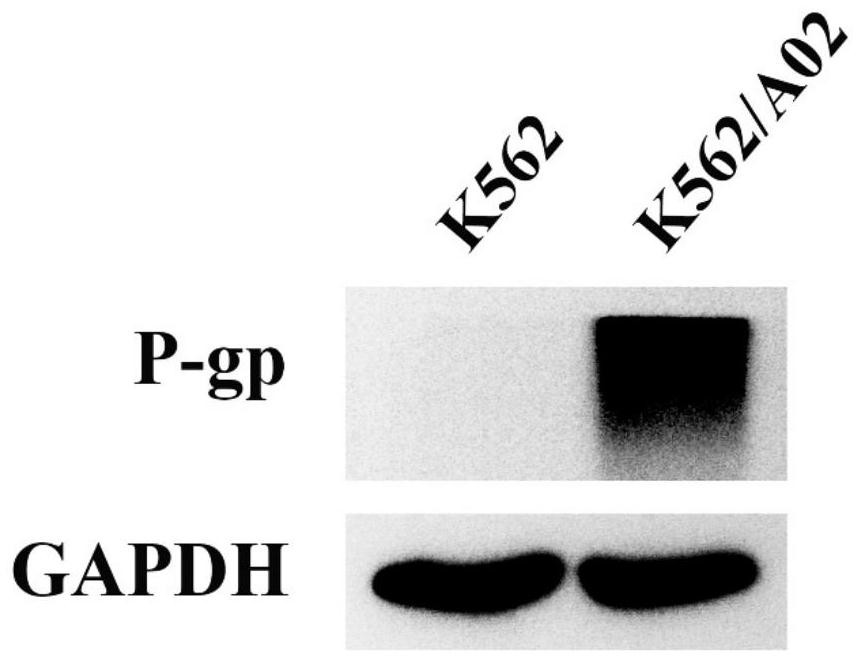
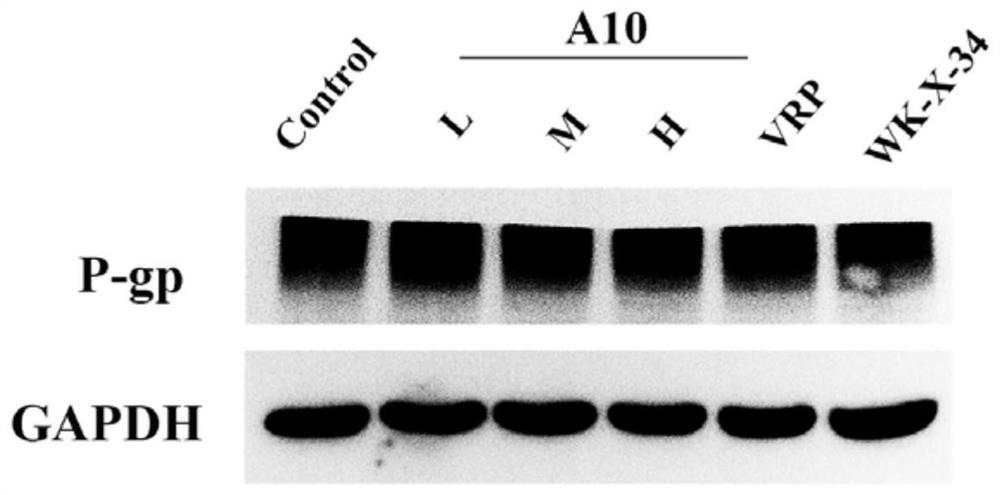
N-phenyl alkoxy dibenzazepine compound as well as preparation method and medical application thereof
A technology of phenylalkoxydibenzoazepines and compounds, which is applied in the field of N-phenylalkoxydibenzoazepines compounds, can solve problems such as low activity of Bifendate, and achieve excellent P-glycoprotein Inhibitory effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0078] 2-(4,10-Dimethoxy-6,8-dihydro-7H-[1,3]dioxo[4',5':3,4]benzo[1,2-c] [1,3]dioxo[4',5':5,6]benzo[1,2-e]azepin-7-yl)phenyl)ethan-1-ol (A1)
[0079] Step 1: Dissolve biphenyl diester (5g, 12mmol) in 200mL tetrahydrofuran, cool to 0°C in ice bath, add LiAlH in batches 4 (1.357g, 36mmol), naturally warmed to room temperature for reaction. The reaction was monitored by TLC. After the reaction was completed, the ice bath was cooled to 0°C, and 2 mL of water, 6 mL of 15% NaOH aqueous solution and 2 mL of water were added to the reaction solution in turn, stirred (off-white suspension) for 10 min, and an appropriate amount of anhydrous sodium sulfate was added and stirred for 30 minutes. Suction filtration after -50min, and the filter cake was washed with methanol several times. The filtrate was concentrated under reduced pressure, dispersed with water, extracted with ethyl acetate, the organic layer was washed with saturated sodium chloride solution, dried over anhydrous sodium...
Embodiment 2
[0083] 7-(4-(2-Bromoethyl)phenyl)-4,10-dimethoxy-7,8-dihydro-6H-[1,3]dioxoxa[4',5' :3,4]benzo[1,2-c][1,3]dioxanone[4',5':5,6]benzo[1,2-e]azepine (A2 )
[0084] Step 1-3: Obtain A1 according to the method of Step 1-3 described in Example 1.
[0085] Step 4: 2-(4,10-Dimethoxy-6,8-dihydro-7H-[1,3]dioxo[4',5':3,4]benzo[1,2 -c][1,3]dioxo[4',5':5,6]benzo[1,2-e]azepin-7-yl)phenyl)ethan-1-ol ( A1) (500 mg, 1.08 mmol) was dissolved in 20 mL of dichloromethane, then phosphorus tribromide (123.5 μL, 1.3 mmol, density: 2.85 g / mL) was added, 0.2 mL of pyridine was added after five minutes, and a metal bath was added under nitrogen protection. The temperature was gradually raised to 45°C, and condensed to reflux. After 2 hours, the reaction of the starting materials was monitored by TLC, and the reaction was completed. Water was added to disperse, the reaction solution was extracted with dichloromethane, the organic layer was washed with saturated sodium bicarbonate and distilled water...
Embodiment 3
[0087] 4-(4,10-Dimethoxy-6,8-dihydro-7H-[1,3]dioxetane[4',5':3,4]benzo[1,2- c][1,3]dioxolane[4',5':5,6]benzo[1,2-e]azepin-7-yl)benzyl alcohol (A3)
[0088] Following the similar method of step 1-3 described in Example 1, use intermediate product III (500 mg, 1.0 mmol), 2-aminobenzyl alcohol (615.7 mg, 5.0 mmol), triethylamine (1.43 mL, 10 mmol) to react to obtain white The title compound (315 mg, 68.2%).
[0089] 1 H NMR (400MHz, DMSO-d 6 )δppm: 7.16-7.07(m, 2H), 6.97-6.88(m, 2H), 6.74(s, 2H), 6.02(d, J=1.0Hz, 2H), 5.96(d, J=1.0Hz, 2H) ), 4.43(d, J=12.9Hz, 2H), 4.34(d, J=5.5Hz, 2H), 3.78(s, 6H), 3.52(d, J=12.8Hz, 2H); ESI-MS: m / z 450.1[M+H] + .
PUM
| Property | Measurement | Unit |
|---|---|---|
| Density | aaaaa | aaaaa |
| Density | aaaaa | aaaaa |
| Density | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com