Dibenzazepine compound used in organic layer of OLED device and organic electroluminescent device
A benzonitride and compound technology, which is applied in the field of organic electroluminescent materials, can solve the problems of small lateral resistance, color crosstalk, and decrease, and achieve the effects of large lateral resistance, low operating voltage, and improved color rendering effect.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0088] The second aspect of the present invention also provides a synthesis method of the dibenzoazepine compound, comprising the following steps:
[0089] S1, in an inert atmosphere, dissolve compound a and organoboron reagent b in a solvent, add potassium tert-butoxide, palladium acetate, and 2-(dicyclohexylphosphine)-biphenyl, and heat to react to obtain compound c;
[0090]
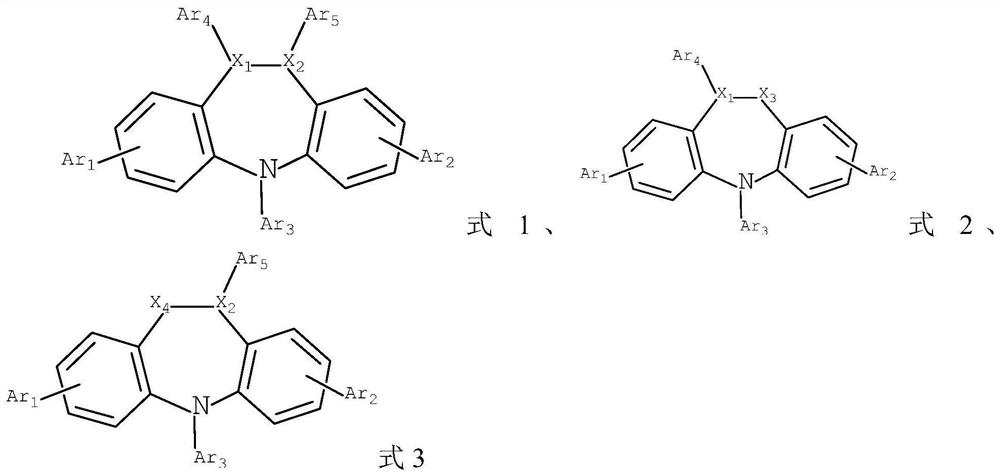
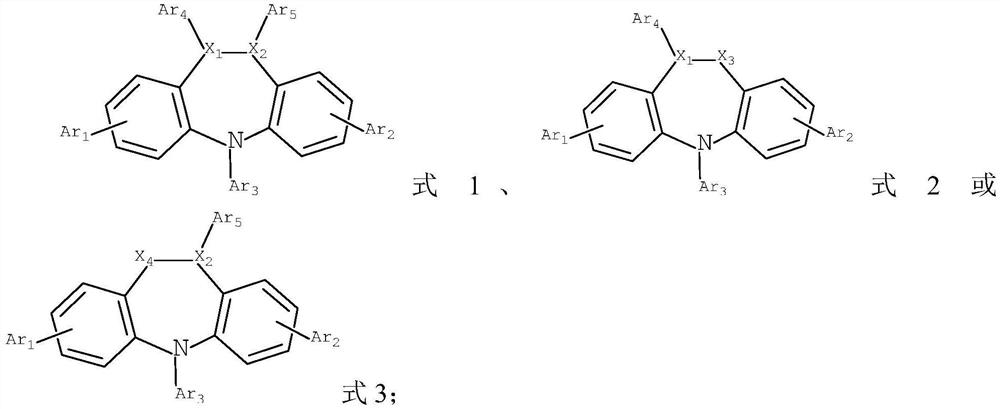
[0091] S2, in an inert atmosphere, dissolve compound c and amine compound d in a solvent, add tetrakis(triphenylphosphine)palladium and potassium carbonate aqueous solution, heat and reflux for reaction, and obtain the compound shown in formula 1 or formula 2 or formula 3;
[0092]
[0093] Among them, R represents the removal of the group -L-NAr in formula 1 6Ar 7 Structures other than Formula 2 remove the group -L-NAr 6 Ar 7 Remove the group -L-NAr in structures other than formula 3 6 Ar 7 outside the structure;
[0094] X is Br or I;
[0095] n is an integer of 1-3;
[0096] L. Ar 6 ...
Synthetic example 1
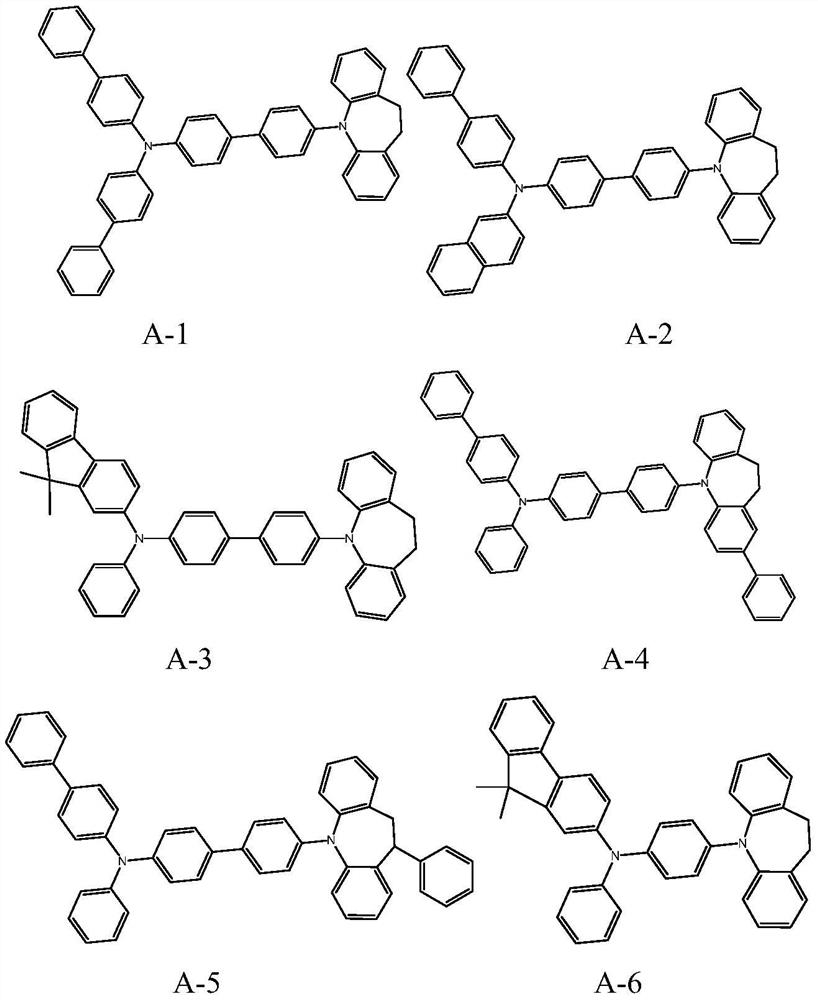
[0119] Synthesis of compound A-1
[0120] In the reaction flask, add 4-bromophenylboronic acid [CAS: 5467-74-3] (70 mg, 0.35 mmol), compound iminodibenzyl [CAS: 494-19-9] (132 mg, 0.68 mmol), tert-butyl Potassium alkoxide (112mg, 1mmol), palladium acetate (10mg), 2-(dicyclohexylphosphine)-biphenyl (30mg) and 10ml of toluene were added to 80°C for 4 hours under nitrogen protection, cooled, and the solvent was removed, crude The product was subjected to silica gel column chromatography to obtain intermediate compound A-1-1 as a solid, 92.6 mg, with a yield of 93%.
[0121] In the reaction flask, add compound A-1-1 (63mg, 0.2mmol), N,N-bis(4-biphenyl)-N-(4-bromophenyl)amine [CAS: 499128-71-1 ] (119g, 0.25mmol), tetrahydrofuran (5ml), tetrakis(triphenylphosphine) palladium (20mg), potassium carbonate aqueous solution (2mol / l, 2mL), heated under reflux overnight under nitrogen protection. Stop the reaction, extract three times with dichloromethane, combine the organic phases, and...
Synthetic example 2
[0128] Synthesis of compound B-1
[0129] In a reaction flask, add bromobenzene (54.5mg, 0.35mmol), hydroxy-substituted iminodibenzyl (143mg, 0.68mmol), potassium tert-butoxide (112mg, 1mmol), palladium acetate (10mg), 2-(bicyclic Hexylphosphine)-biphenyl (30mg) and 10ml of toluene were added to 80°C for 4 hours under nitrogen protection and reacted for 4 hours, cooled to obtain compound B-1-1; then added hot HBr to replace, remove the solvent, and the crude product was used on a silica gel column The intermediate compound B-1-2 was obtained by chromatography as a solid 97 mg, with a yield of 80%.
[0130] Referring to the synthesis process of compounds A-1-1 and A-1 in Synthesis Example 1, under the same conditions, the reactants were replaced to obtain compound B-1, 92 mg, with a yield of 45%.
[0131] Structure Characterization:
[0132] Mass Spectrum m / z: 590.27;
[0133] Element content (%): C 44 h 34 N 2 . C, 89.46%; H, 5.80%; N, 4.74%;
[0134] 1 H NMR: δ2.92 (...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com