Pharmaceutical preparation composition containing WEE1 inhibitor and preparation method thereof
A technology for pharmaceutical preparations and compositions is applied in the field of pharmaceutical compositions containing WEE1 inhibitors as active ingredients and their preparation fields, and can solve the problems of poor water solubility and low bioavailability of compounds.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1~2
[0027] The pharmaceutical composition of the present invention, the preferred formula composition is as follows (%, w / w):
[0028]
[0029]
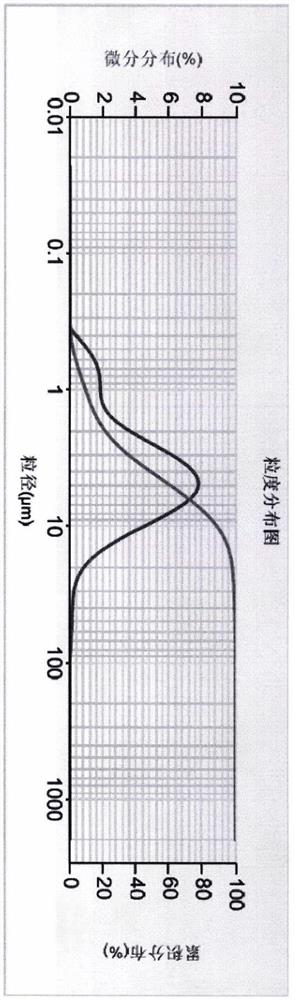
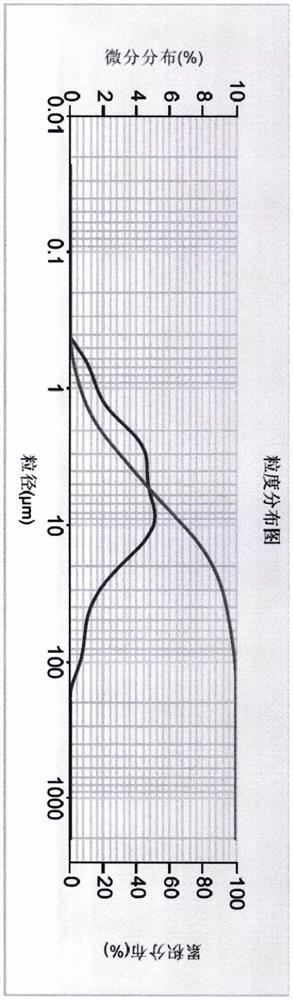
[0030] Particle size distribution of compounds of formula (I):
[0031] Particle size distribution Example 1 Example 2 D50 4.047μm 8.886μm D90 10.428μm 51.124μm
[0032] The preparation process is as follows:
[0033] 1) mix the compound of formula (I), microcrystalline cellulose PH102, lactose F100, sodium starch glycolate and magnesium stearate (internally added);
[0034] 2) Take the mixed material and add it to the hopper of the dry granulator for granulation. The feeding frequency is 20Hz, the tableting frequency is 10Hz, the granulation frequency is 10Hz, and the oil pressure is 40-50kg / cm 2 , the aperture of the secondary screen mesh is 1.0mm;
[0035] 3) Mix the prepared granules with the external materials evenly, press the tablet with a rotary tablet machine, and perform film coating. ...
Embodiment 3~4
[0037] The pharmaceutical composition of the present invention, the preferred formula composition is as follows (%, w / w):
[0038] Raw materials Proportion Compounds of formula (I) 40.0 Microcrystalline Cellulose PH101 44.0 Croscarmellose sodium 3.0 Povidone K30 5.0 Poloxamer 188 5.0 Croscarmellose sodium (extra) 2.0 Magnesium stearate (extra) 1.0 sum 100.0
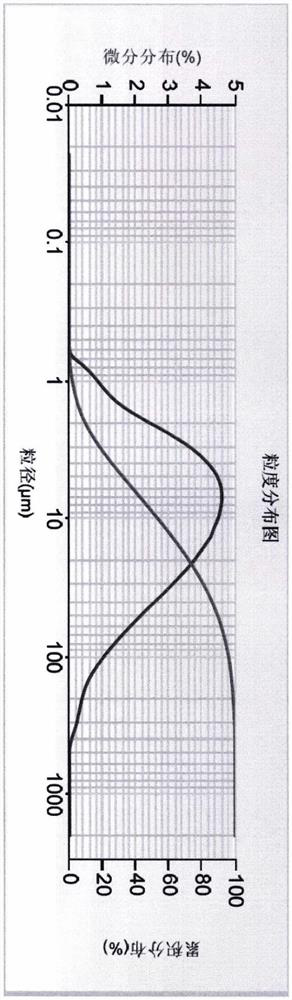
[0039] Particle size distribution of compounds of formula (I):
[0040] Particle size distribution Example 3 Example 4 D50 5.785μm 10.697μm D90 25.863μm 101.713μm
[0041] The preparation process is as follows:
[0042] 1) adding poloxamer 188 and povidone K30 to a certain amount of purified water, stirring and dissolving to obtain a binder solution with a concentration of 20% of povidone K30;
[0043] 2) adding the compound of formula (I), microcrystalline cellulose PH101 and croscarmellose sodium into the wet granulator h...
experiment example 1
[0048] Determination of the Dissolution Profile of the Invention
[0049] The samples of the above-mentioned examples were taken, and the dissolution curve was determined by the second method of the Dissolution and Release Determination Method 0931 of the Fourth General Principles of the Chinese Pharmacopoeia 2020 Edition. The dissolution method is: paddle method, 0.1N HCl solution and pH4.5 phosphate buffer, 900ml, 75rpm, 37±0.5°C, sampling time 10, 15, 30 and 60min. The dissolution profile is attached Figures 5 to 6 . It can be seen that in the two dissolution media, the dissolution of Example 1 and Example 3 prepared by micronized raw materials are significantly faster than those of Example 2 and Example 4.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com