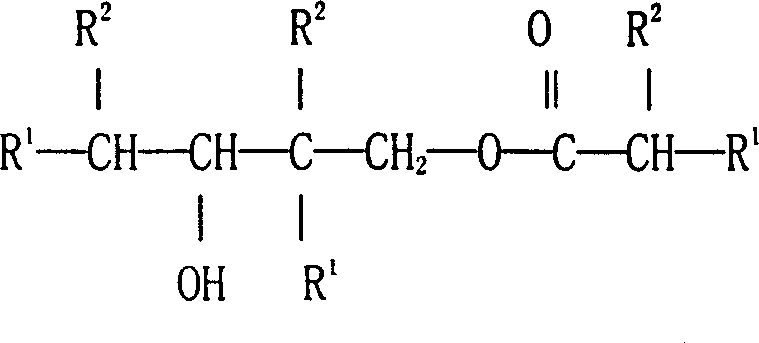
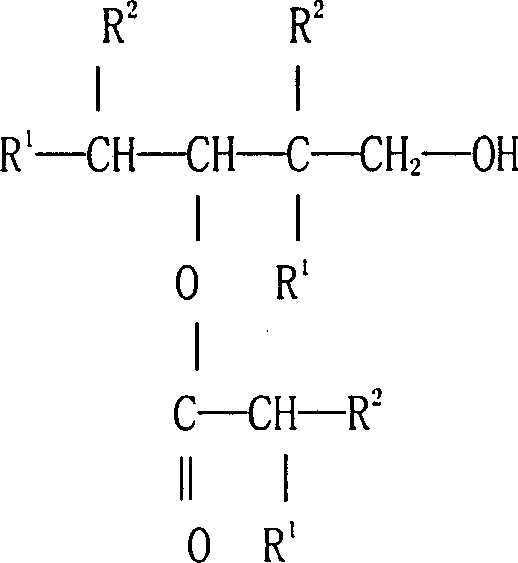Preparation process of 2,2,4-trimethyl-1,3-pentanediol mono-sio butyrate
A technology of pentanediol monoisobutyrate and preparation process is applied in the field of preparation technology of 2,2,4-trimethyl-1,3-pentanediol monoisobutyrate and can solve the intermediate steps of the reaction Many problems, such as prolonged reaction time, low reaction selectivity, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] In a 500ml reactor equipped with a reflux condenser and a stirring device, add 6g of a 45% sodium hydroxide aqueous solution, then slowly add 200g of isobutyraldehyde for condensation reaction, control the reaction temperature at 20-30°C, and the reaction time for 3h. Then the temperature was raised to 60°C, and the reaction was carried out at constant temperature for 2h.
[0032]Analysis of the mixed product showed that the conversion rate of isobutyraldehyde was 70%, and 86% of 2,2,4-trimethyl-1,3-pentanediol monoisobutyrate, 2,2,4- Trimethyl-1,3-pentanediol 6%, 2,2,4-trimethyl-1,3-pentanediol diisobutyrate 2%, isobutanol 1.5%, isobutyl isobutyrate Esters 2.5%, other components 2%.
[0033] The above mixed solution is subjected to vacuum rectification under the condition of vacuum pressure of -0.097MPa to obtain a product with a purity of 98.2%.
Embodiment 2
[0035] Experimental device and steps are the same as Example 1. First add 5g of dibutyltin dilaurate, then slowly add 200g of isobutyraldehyde for reaction, control the reaction temperature by adjusting the feeding speed and the amount of cooling water, 30-40°C, and the reaction time is 4h. Then the temperature was raised to 70°C, and the reaction was carried out at constant temperature for 6h.
[0036] The content of the reaction product was analyzed: the conversion rate of isobutyraldehyde was 60%. 90% of 2,2,4-trimethyl-1,3-pentanediol monoisobutyrate was obtained in the reaction product, other products included 4% of 2,2,4-trimethyl-1,3 -Pentanediol, 2% of 2,2,4-trimethyl-1,3-pentanediol diisobutyrate, 4% of other components such as isobutanol.
Embodiment 3
[0038] Experimental device and steps are the same as Example 1. Add 5g of calcium hydroxide to the reactor, dropwise add 200g of isobutyraldehyde as the raw material for reaction, control the reaction temperature of the first step to 20-30°C, and the reaction time to 2h. Then the temperature was raised to 80° C., and the reaction was carried out at constant temperature for 10 h.
[0039] The experimental results are as follows: the conversion rate of isobutyraldehyde is 86%, and 91% of 2,2,4-trimethyl-1,3-pentanediol monoisobutyrate and 2.5% of 2,2,4 - trimethyl-1,3-pentanediol, 1.5% diester (2,2,4-trimethyl-1,3-pentanediol diisobutyrate), 1% isobutanol, Other components 4%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com