Hexavalent amino amidate derivative with function of inhibiting blood vessel growth activity
An amino, carboxamide technology, applied in the direction of organic active ingredients, cardiovascular system diseases, medical preparations containing active ingredients, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
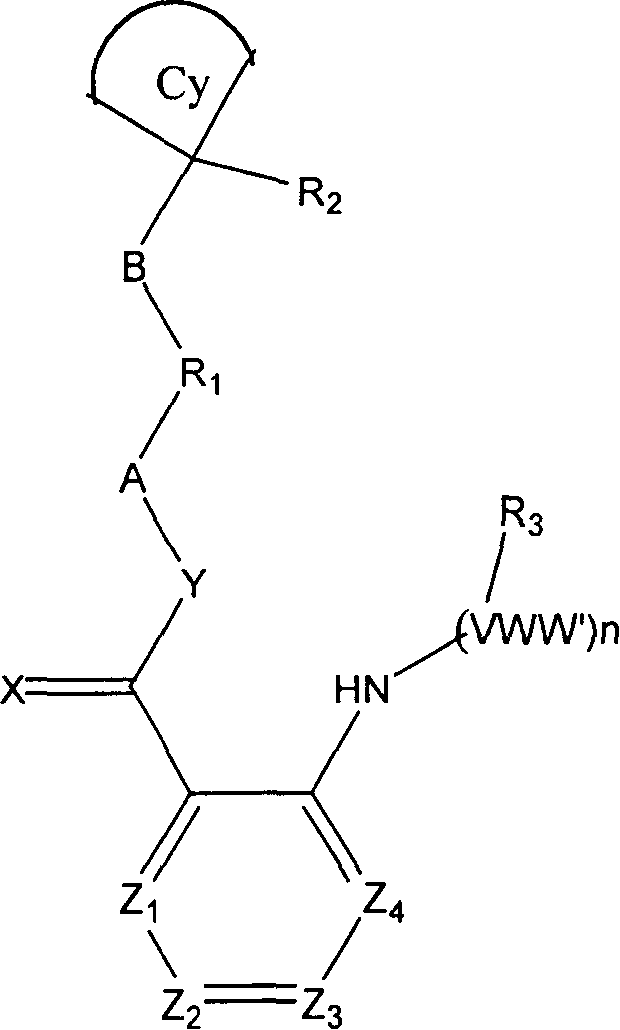
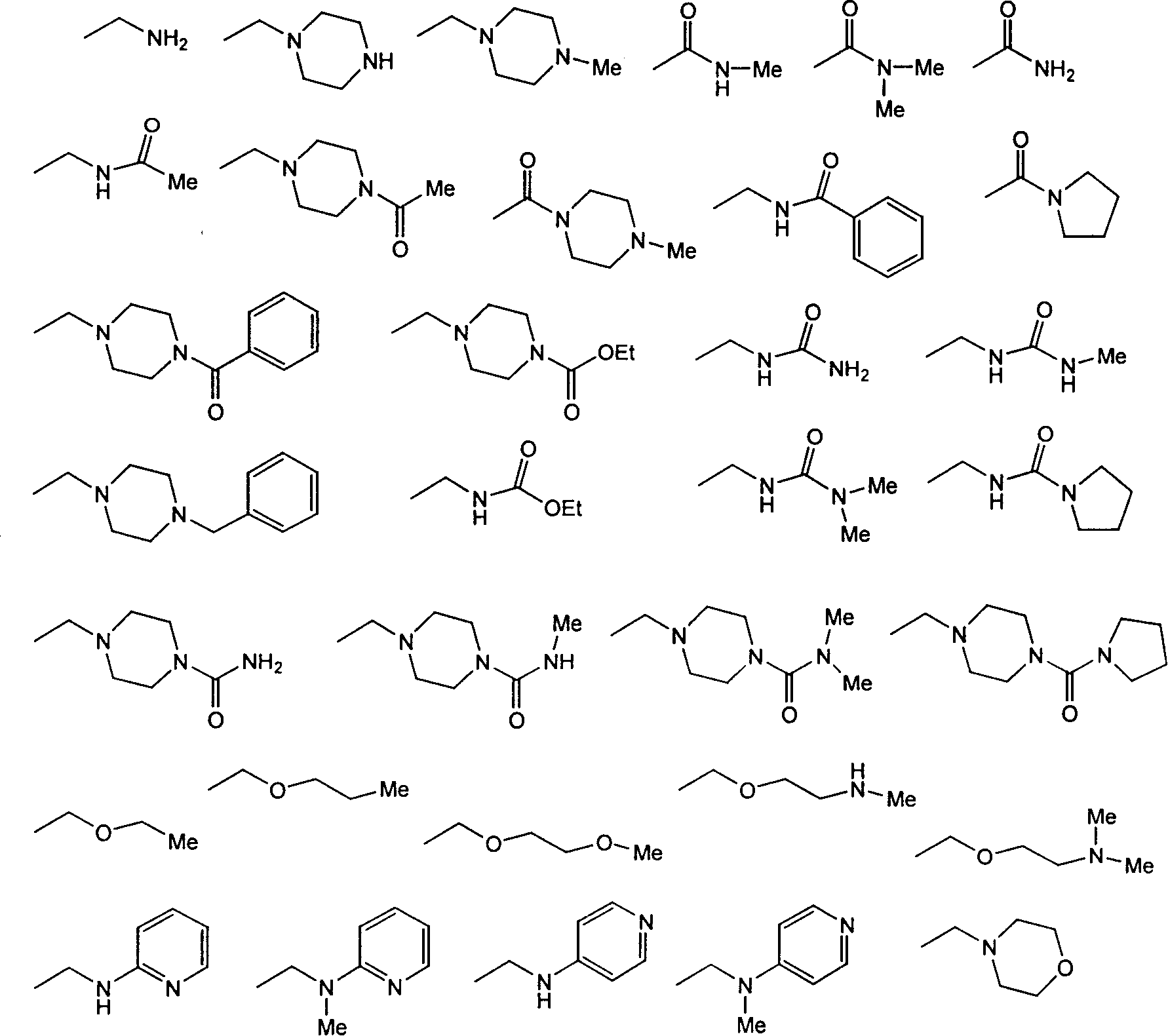
[0139] N-[4-(cyanocyclobutyl)phenyl]{2-[(4-pyridylmethyl)amino]phenyl}carboxamide
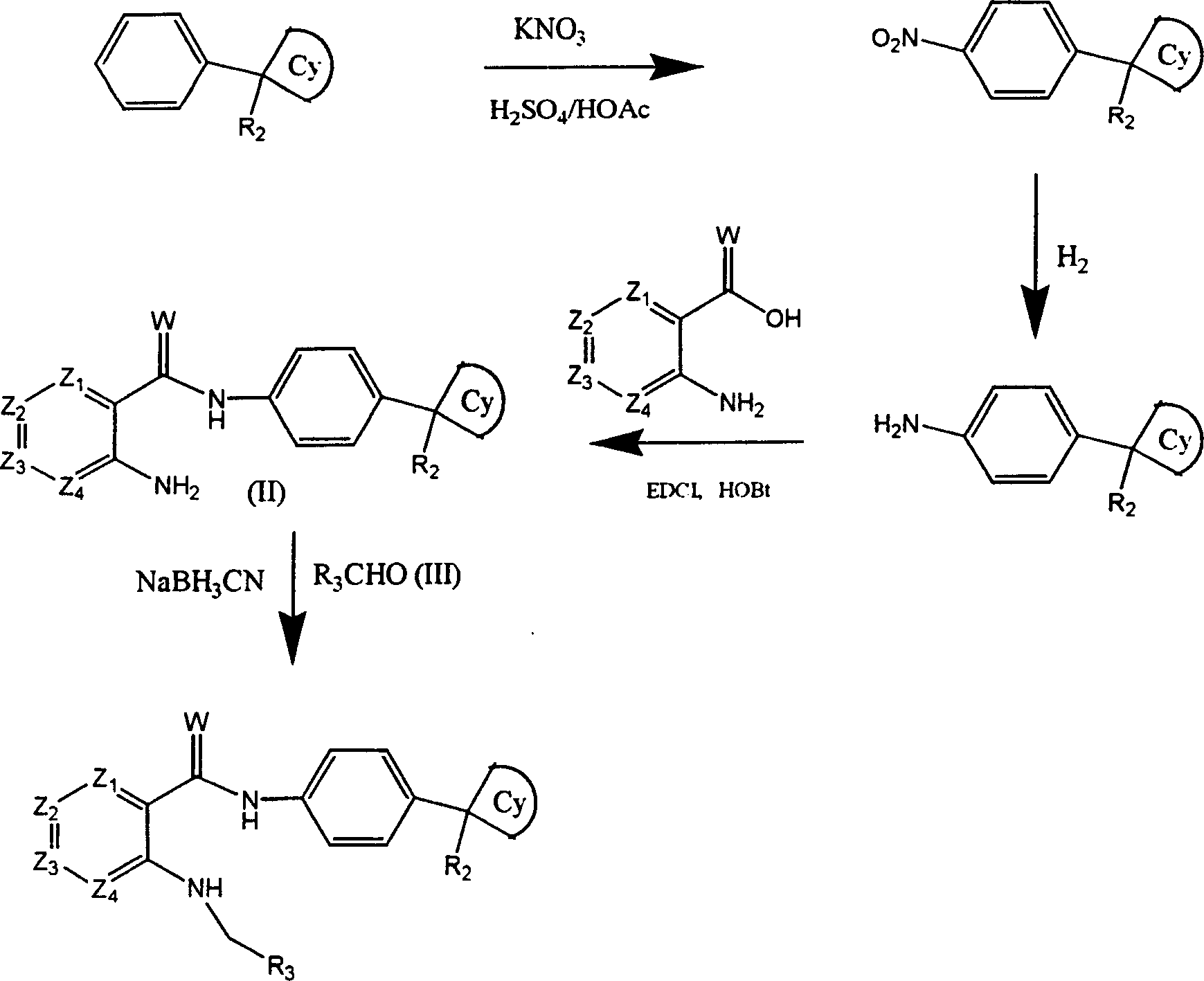
[0140] A: 1-(4-Aminophenyl) cyclobutyl nitrile
[0141] Mix 1-phenylcyclobutyl nitrile (5g), acetic acid (15ml), H 2 SO 4 (10ml) and KNO 3 (1.1 molar ratio), stir at 0°C for 20 minutes, then warm to room temperature and stir for another 2 hours. Then the mixture was poured into ice cubes and stirred until the ice cubes were completely dissolved, and a solid precipitated out, filtered with suction and recrystallized from ethanol to obtain 1-(4-nitrophenyl)cyclobutyronitrile. Mix 2 g of the above product with palladium-carbon (800 mg, 10%) and ethanol (100 ml), hydrogenate at atmospheric pressure for 1 hour, then filter with suction, and evaporate the filtrate to obtain 1-(4-aminophenyl)cyclobutane Base nitrile. Mass spectrum (M+1), 172, the final product is purified for use in the next step.
[0142] B. 2-Aminophenyl-N-[4-(cyanocyclobutyl)phenyl]carboxamide
[0143] A mixture of anthranilic acid (1....
Embodiment 2
[0147] N-[4-(cyanocyclopropyl)phenyl]{2-[(4-pyridylmethyl)amino]phenyl}carboxamide
[0148] This compound was prepared by a method similar to Example 1, starting from 1-phenylcyclopropyl nitrile. Mass spectrum: (M+1), 369
Embodiment 3
[0150] N-[4-(cyanocyclopentyl)phenyl]{2-[(4-pyridylmethyl)amino]phenyl}carboxamide
[0151] This compound was prepared using a method similar to Example 1, starting from 1-phenylcyclopentyl nitrile. Mass spectrum: (M+1), 395
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com