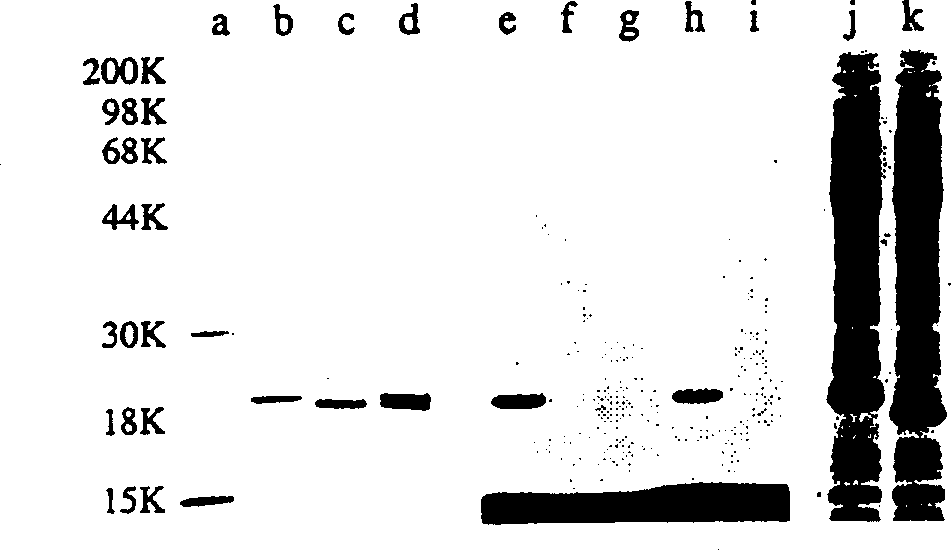
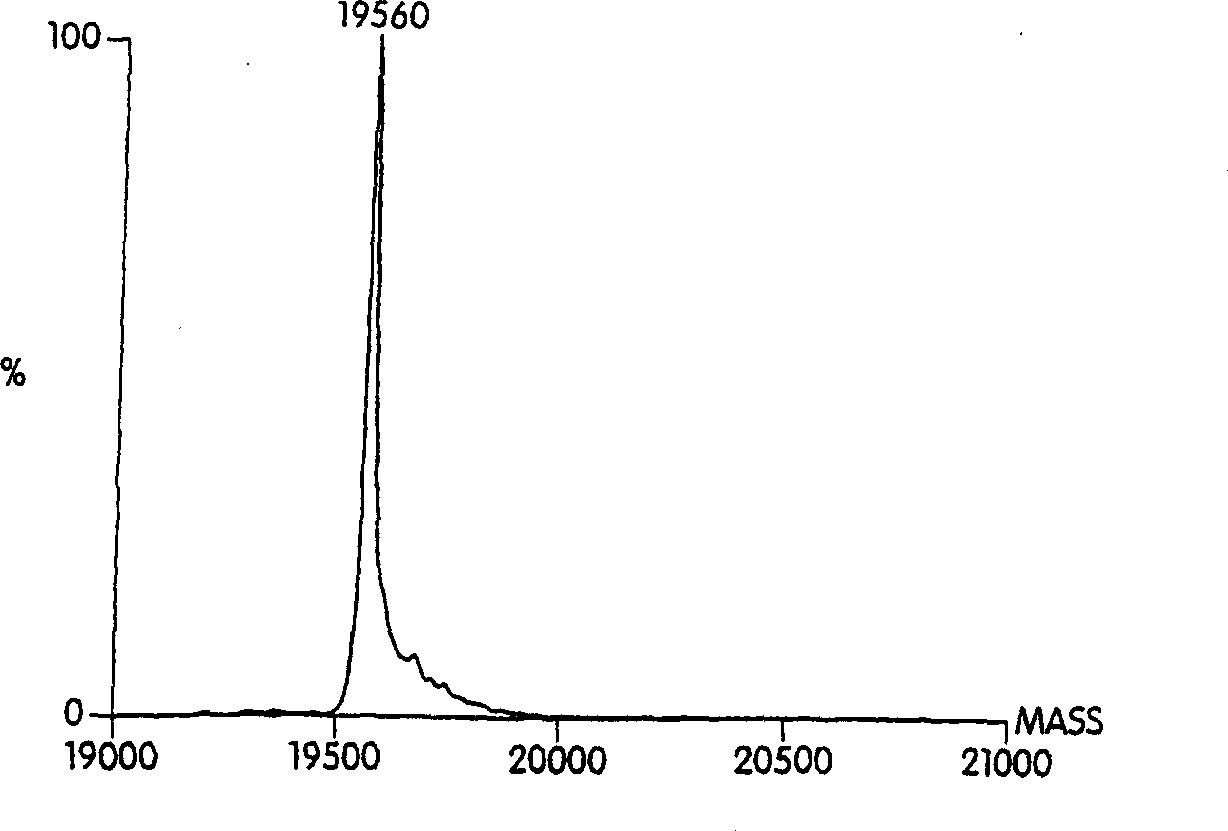
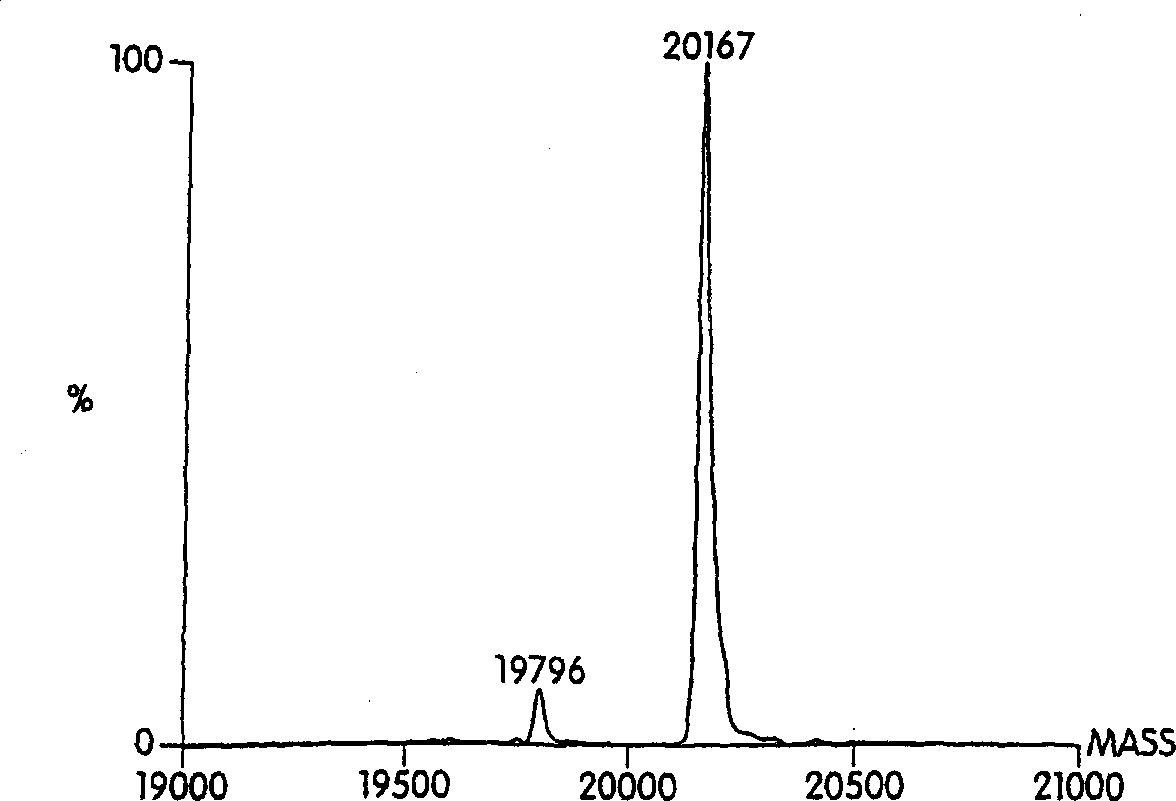
Hydrophobically-modified protein compsns, and methods
A hydrophobic group and protein technology, which is applied in the preparation method of peptides, drug combinations, peptide/protein components, etc., can solve the problems of negative impact on the application of preparations and complicated experiments
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment approach
[0012] Another embodiment is an isolated protein of the form A-Cys-[Sp]-B-X, wherein A is a hydrophobic group, Cys is cystine or its functional equivalent, [Sp] is an optional spacer peptide sequence, and B is A protein comprising a plurality of amino acids, including at least one optional spacer peptide sequence; and X is an optional other hydrophobic group attached to the protein.
[0013] The isolated protein may be an extracellular signaling protein, preferably a hedgehog protein. The protein may be modified with at least one hydrophobic group at at least one other amino acid. In another embodiment, the protein is in contact with a vesicle selected from the group consisting of cell membranes, micelles and liposomes.
[0014] Another aspect of the invention is an isolated protein having a C-terminal amino acid and an N-terminal thioproline group formed by the reaction of an aldehyde with the N-terminal cystine of the protein . Yet another aspect of the invention is an is...
Embodiment 4
[0176] Preferred sterols and sterol esters suitable for use in preparing multimeric protein complexes include cholesterol, cholestanol, cholesterol sulfate, and other cholesterol analogs and derivatives. The fact that vesicles can comprise many different lipids and detergents allows for great variability in engineering binding protein-vesicle complexes with desired properties. For example, one can make vesicles that incorporate different kinds of proteins by changing the composition of the lipids in the starting material to produce larger vesicles, or by increasing the percentage of phosphatidylinositol lipids in the vesicles. Ⅶ. Practicality
[0177] In general, the modified proteins described herein can be used to treat conditions that can be treated with unmodified proteins. However, the hydrophobically modified proteins described herein have several significant improvements over the unmodified form. First, their increased potency allows smaller amounts of protein to be u...
Embodiment 1
[0194] The invention will now be described in more detail with reference to the following non-limiting examples. Example 1: Lipid modification of human Sonic hedgehog when expressed in insect cells A. Expression of human Sonic hedgehog
[0195] The cDNA of full-length human Sonic hedgehog (Shh) was subcloned in pBluescript SK + The 1.6 kb EcoRI fragment from (20) (a gift from David Bumcrot of Ontogeny, Inc., Cambridge MA). The 5' and 3' NotI sites, which immediately flank the Shh open reading frame, were added by unique site-elimination mutagenesis using the Pharmacia kit according to the manufacturer's recommended protocol. The 1.4 kb NotI fragment carrying the full-length Shh cDNA was then subcloned into the insect expression vector pFastBac (Life Technologies, Inc.). Using Life Technologies, Inc. The provided procedure produces recombinant baculovirus. The obtained virus is used to generate a high titer virus stock solution. Methods for the preparation and purification...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com