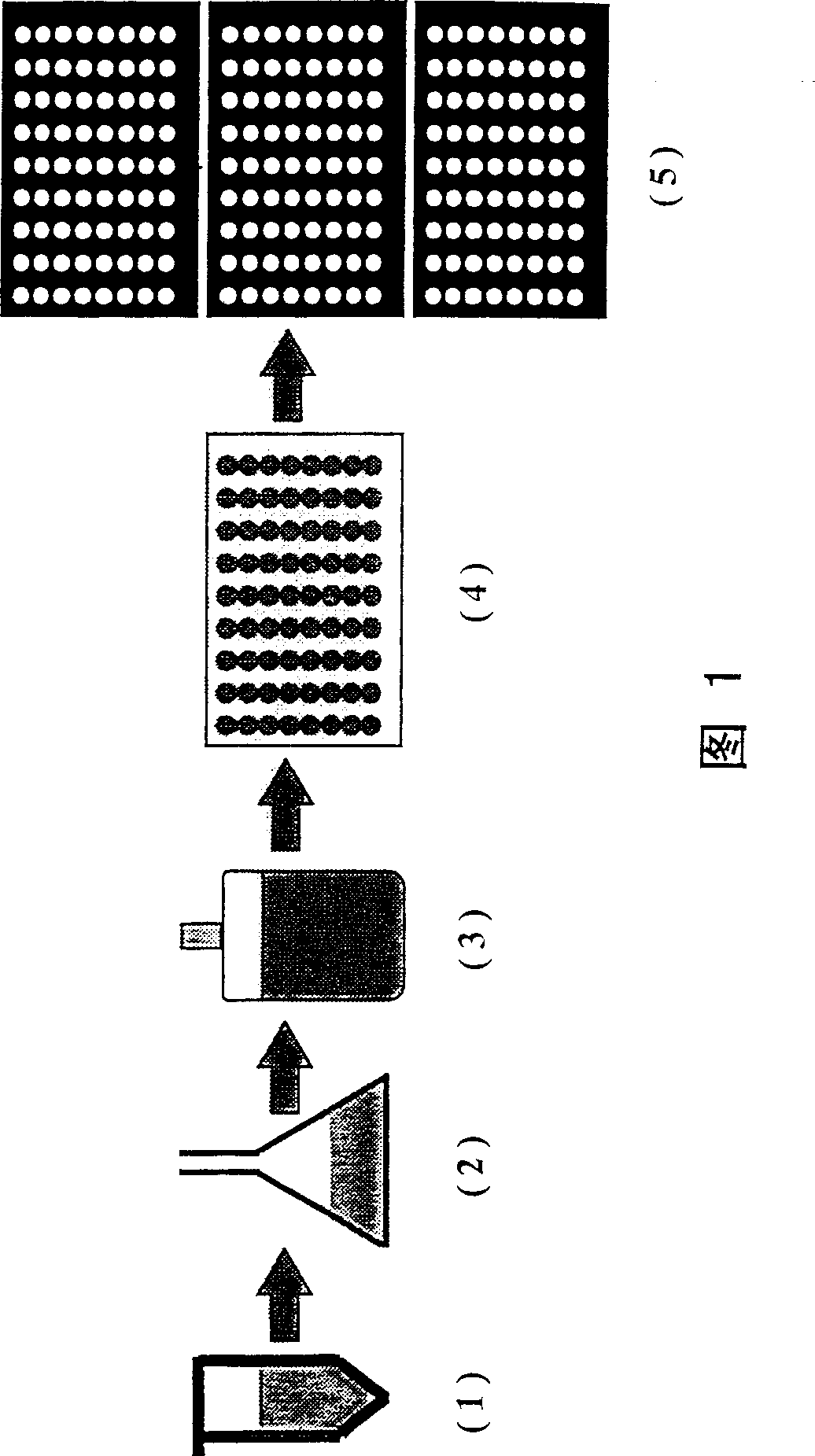
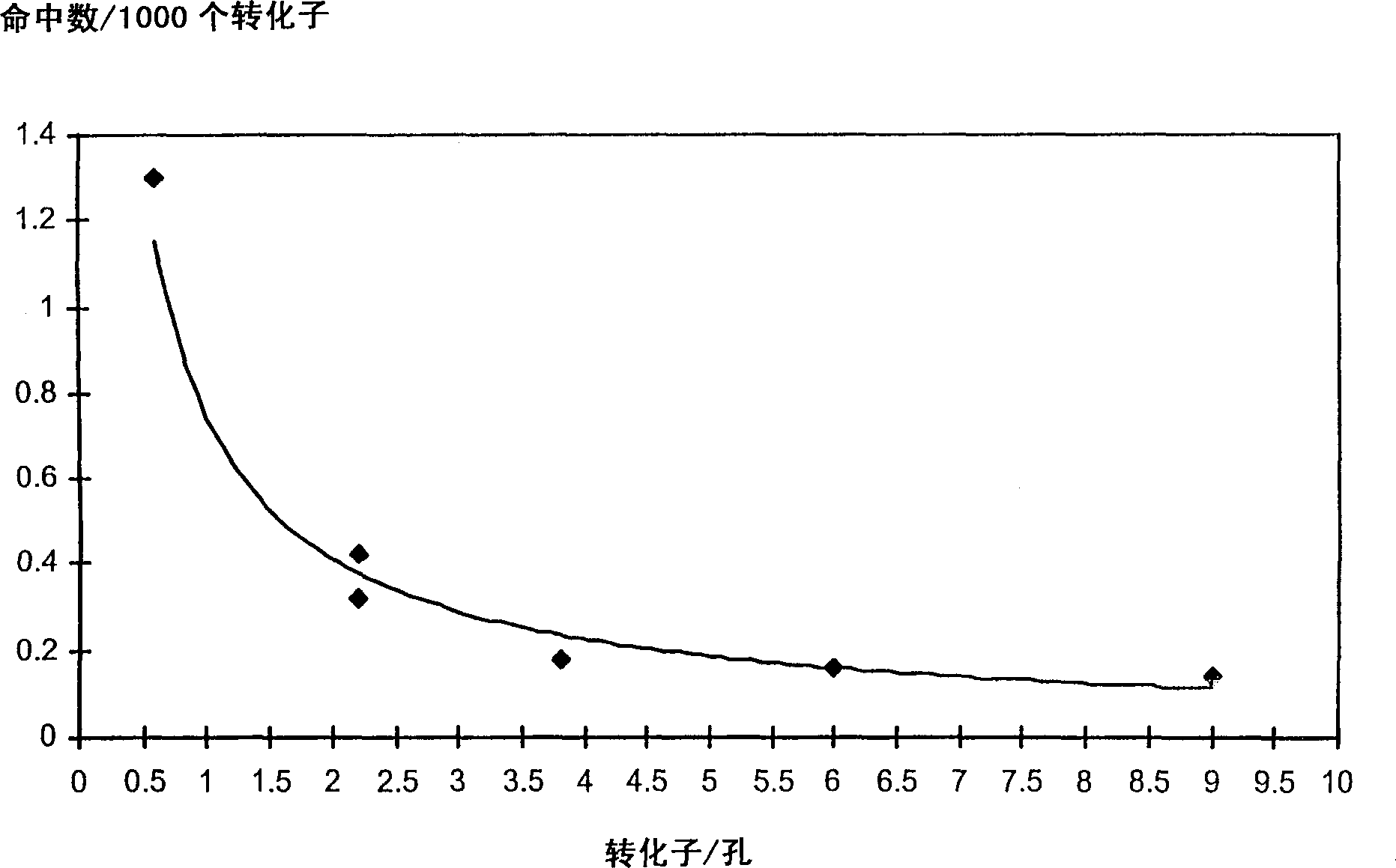
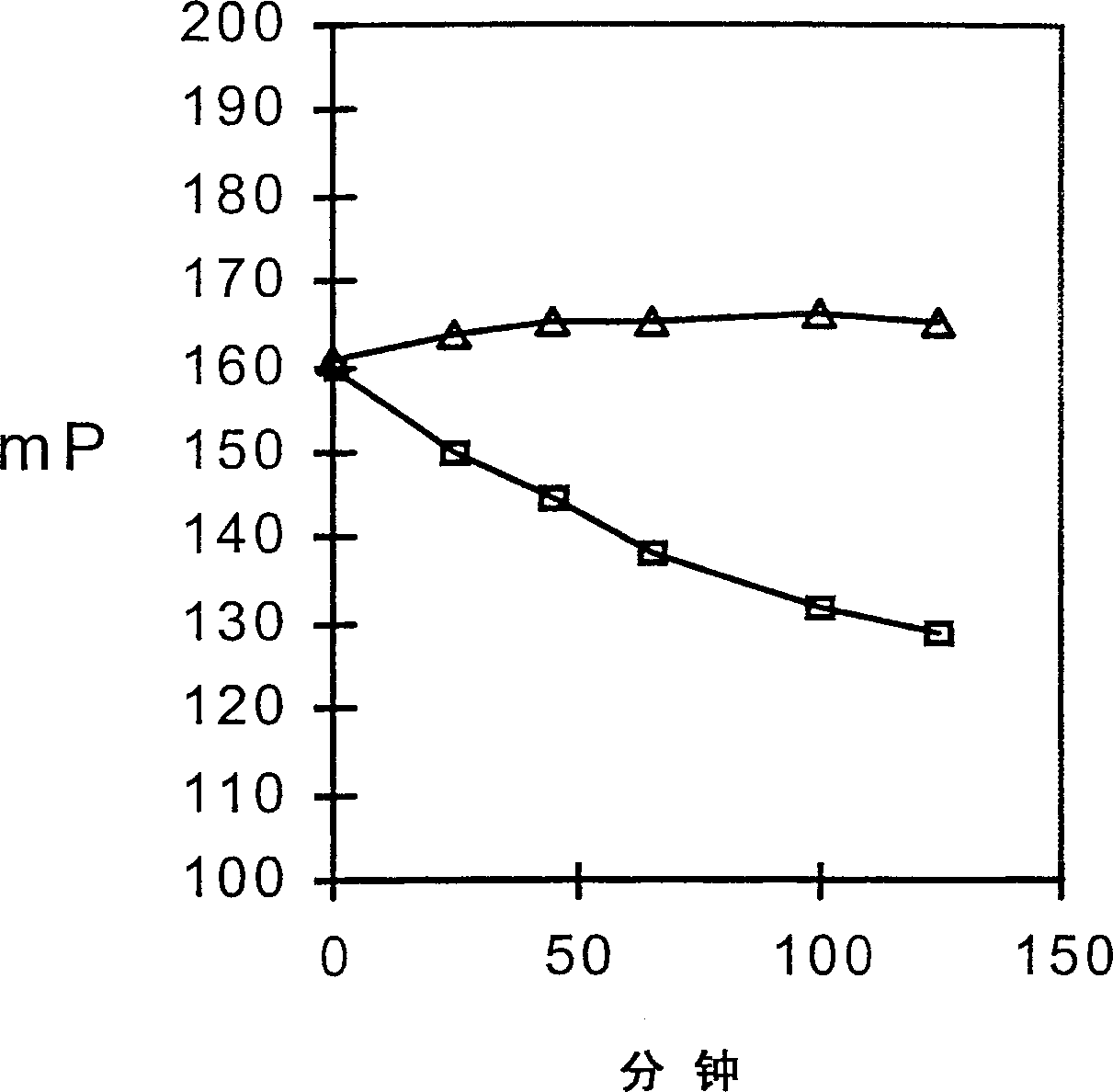
Fluorescence polarization screening method
A technology of fluorescence polarization and fluorescent substances, which is applied in the fields of fluorescence/phosphorescence, biochemical equipment and methods, and material analysis through optical means, and can solve problems such as limited and lack of applications
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0141] The invention is illustrated by the following non-limiting examples: Example 1: Labeled oligoxyloglucan
[0142] The oligoxyloglucan was end-labeled with sulforhodamine as described by Fry et al. (1997). Example 2: Labeling of xyloglucan by partial chemical oxidation
Embodiment 2
[0143] After stirring for 7 hours, xyloglucan (0.50 g) was dissolved in water (60 ml) at 60°C. The solution was cooled to room temperature, and an aqueous solution (10 ml) containing sodium metaperiodate (15 mg) (Merck, Germany) was added. The mixture was left overnight in the dark, and then dialyzed against demineralized water (molecular weight cut-off of 12,000-14,000) and the receiving solution was changed frequently. 25 mg of fluorescein-5-thiosemicarbazide (Molecular Probes, OR, USA) was dissolved in 1 ml of DMF (Sigma, MO, USA), and this orange solution was added to the dialyzed polymer (approximately 60 ml of solution). The solution was stirred in the dark for 18 hours. The polymer was precipitated by adding 96% ethanol (20 ml), filtered on Miracloth (Calbiochem, Germany) to collect the wet polymer, and washed with a mixture of water and ethanol (1:1) until A colorless filtrate was obtained. Finally, the labeled yellow xyloglucan was washed with absolute ethanol and air-dri...
Embodiment 3
[0144] Arabinan (0.50 g) was dissolved in water (10 ml), and an aqueous solution (5 ml) containing sodium metaperiodate (15 mg) was added. The mixture was left in the dark overnight, then diluted with water (15 ml), the solution was dialyzed against water (molecular weight cut-off 12,000-14,000) and the receiving solution was frequently replaced. 25 mg of fluorescein-5-thiosemicarbazide (Molecular Probes, OR, USA) was dissolved in 1 ml of DMF, and this orange solution was added to the dialyzed polymer. The solution was stirred in the dark for 36 hours, and then the solution was divided into two parts. One part was concentrated to a smaller volume. The polymer was precipitated by adding 90% ethanol. It is estimated that the marking degree is 0.7% (from 492nm). Calculate the absorption value). Example 4: Labeling of polygalacturonic acid
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com