A topical nanoparticulate spironolactone formulation
A technology of nano-particles and spironolactone, which is applied in the direction of aerosol delivery, medical preparations with non-active ingredients, medical preparations containing active ingredients, etc., which can solve the adverse effects of product stability, tiny particles and nanoparticles Aggregation and flocculation, narrow particle size range, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0060] Example 1: Preparation of Nanoparticulate Spironolactone as Topical Formulation
[0061] Preparation of Nanoparticle Spironolactone
[0062] Table 1 shows a typical preparation method of nanoparticulate spironolactone incorporated into the crystal structure according to the present invention. Nanoparticle spironolactone can be prepared as follows:
[0063] Combine the aqueous formulation of the stabilizer with water or injection buffer under magnetic stirring until a clear solution is obtained; wet the spironolactone with the appropriate amount of aqueous surfactant to form a slurry; disperse the resulting suspension with high shear Disperse the suspension with magnetic force to eliminate foam; the obtained suspension passes through a high-pressure piston gap homogenizer to obtain a nano-suspension. Use Avestin C5 TM Formulations 1-7 were prepared using Avestin C50 TM Formulations 8-9 were prepared. During homogenization, drug particles are broken up by cavitation ...
Embodiment 2
[0122] Example 2: Size of spironolactone particles before and after storage
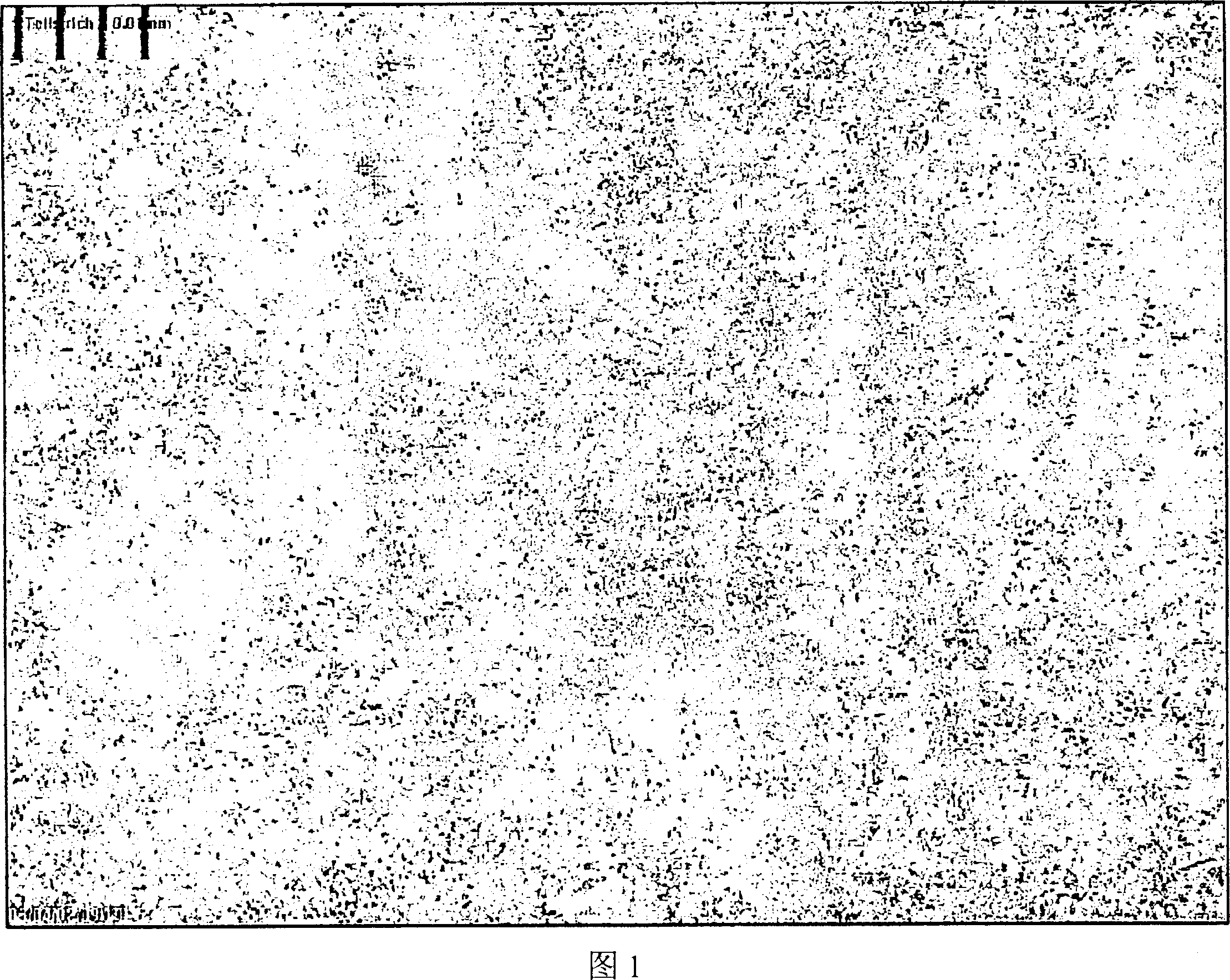
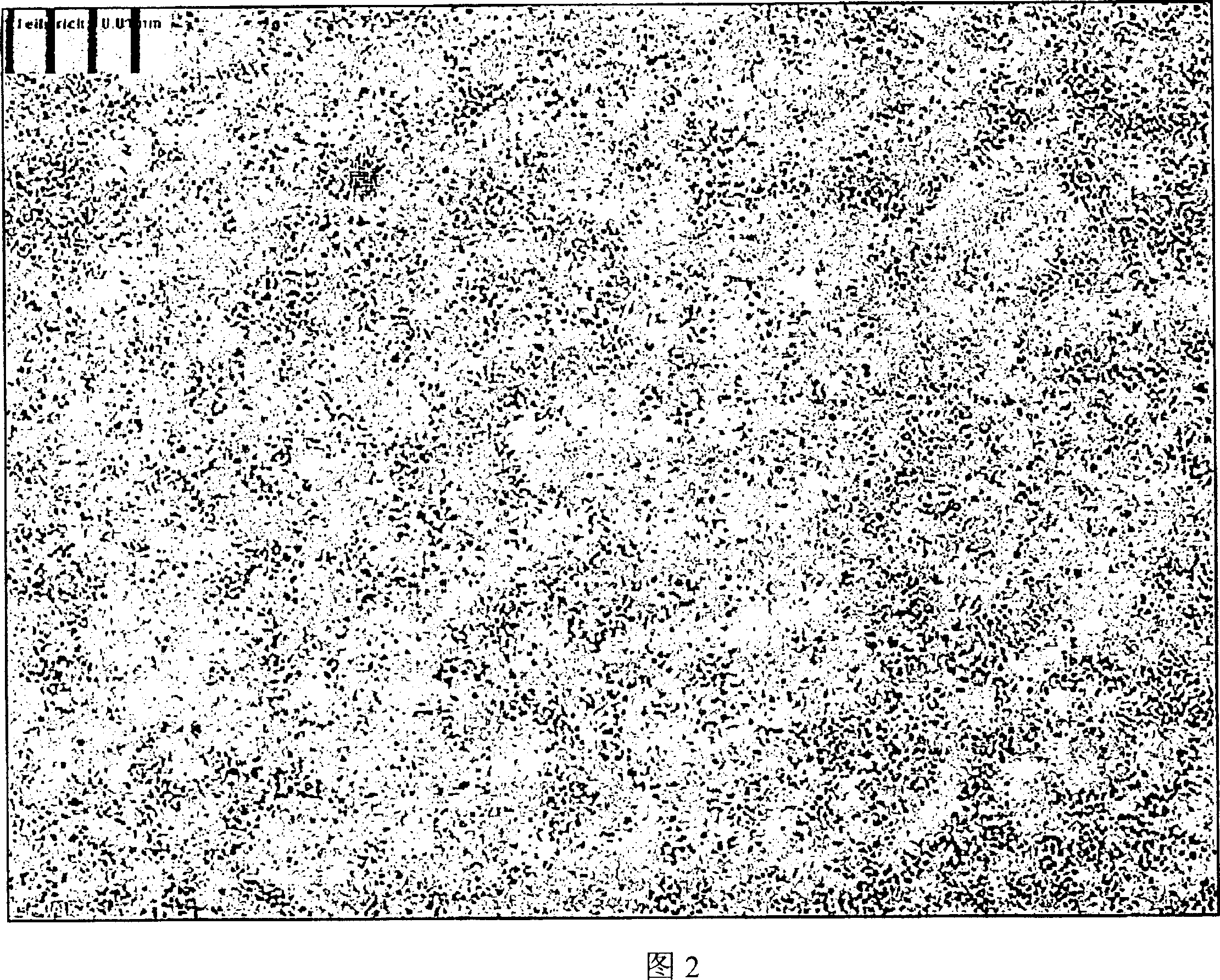
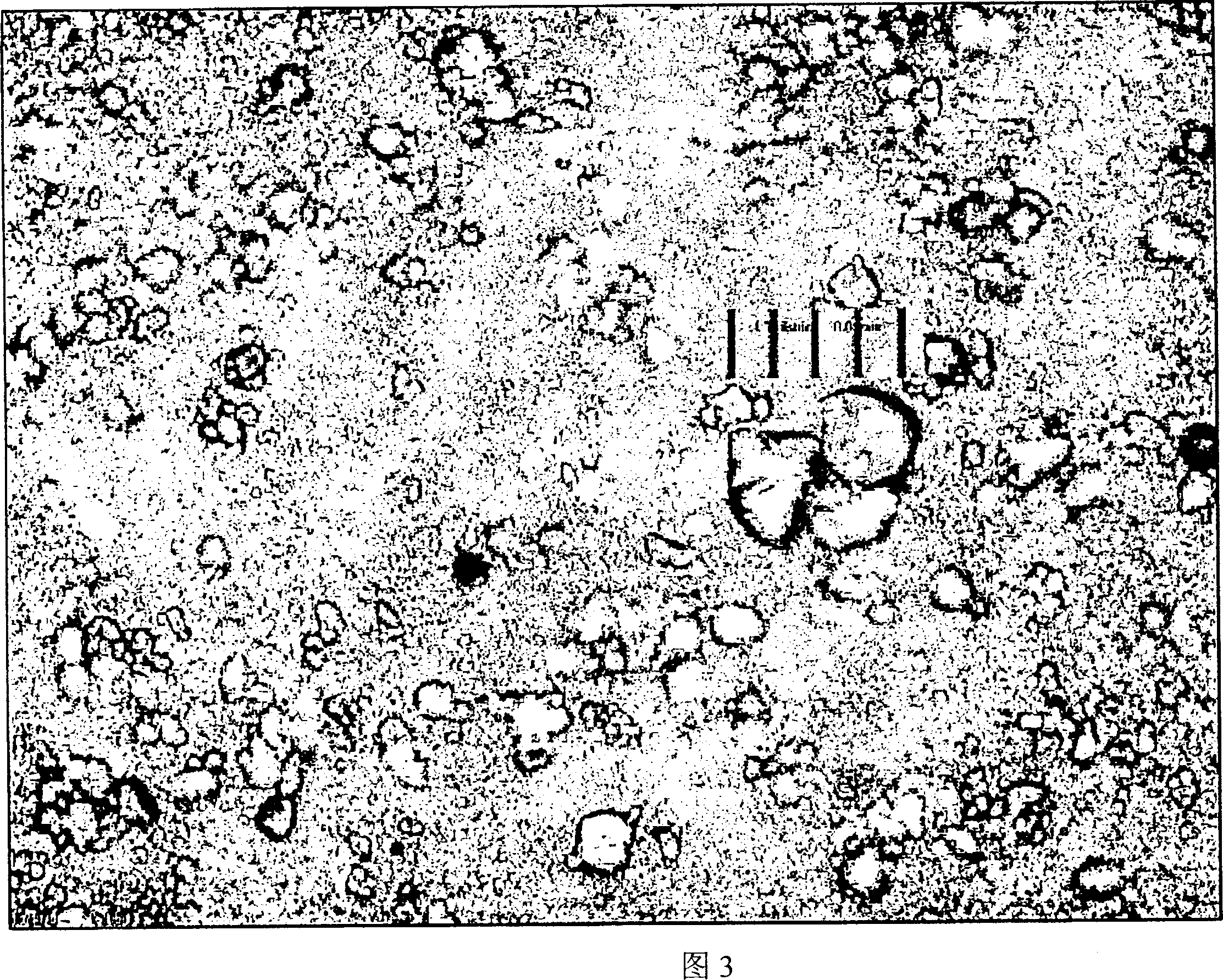
[0123] Figures 1, 2 and 3 are micrographs of spironolactone of the present invention when it is just prepared, after being stored for 7 months, and compared with commercially available spironolactone. The distance between each vertical grid in the figure is 0.01 mm or 10 microns.
[0124] The particles shown in Figures 1 and 2 are so small that they are barely visible under an optical microscope. The particles did not grow any longer after storage for 7 months. In contrast, the commercially available spirosterolactone "Spiroderm" (Figure 3) presents spironolactone crystals as large as 20 microns.
Embodiment 3
[0125] Example 3: Flux Study
[0126] raw material
provider
Crystalip TM Spironolactone nanosuspension 2% by weight
Lot number is 4014-000 / 06atc
Spironolactone
The batch number is 510 / 0
Skye Pharma, Switzerland
Aqueous Cream B.P.
The batch number is 28076
Hillcross, UK
R grade analytically pure
VWR International Ltd., UK
Sodium dihydrogen phosphate dihydrate
The batch number is L298
Merckeurolab
Deionized water
Elga Ltd., UK
Acetonitrile HPLC grade
Rathburns Chemicals Ltd., UK
NBS-Biological, UK
[0127] Methods of Flow Research
[0128] Crystalip TMThe formulation was provided by SkyePharma AG, and the commercially available spironolactone control was no longer used in the experiment, so a standard cream base control was prepared as follows. Briefly, 100 mg of spironolactone powder was accurately weighed an...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
| crystallization temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com