Effective part group in red sage mixture and its delayed release prepn. medicinal use and prepn process
A technology of effective parts and sustained-release preparations, applied in the field of medicine, can solve the problems of forgetting to take, easy to miss, and inconvenient to take.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
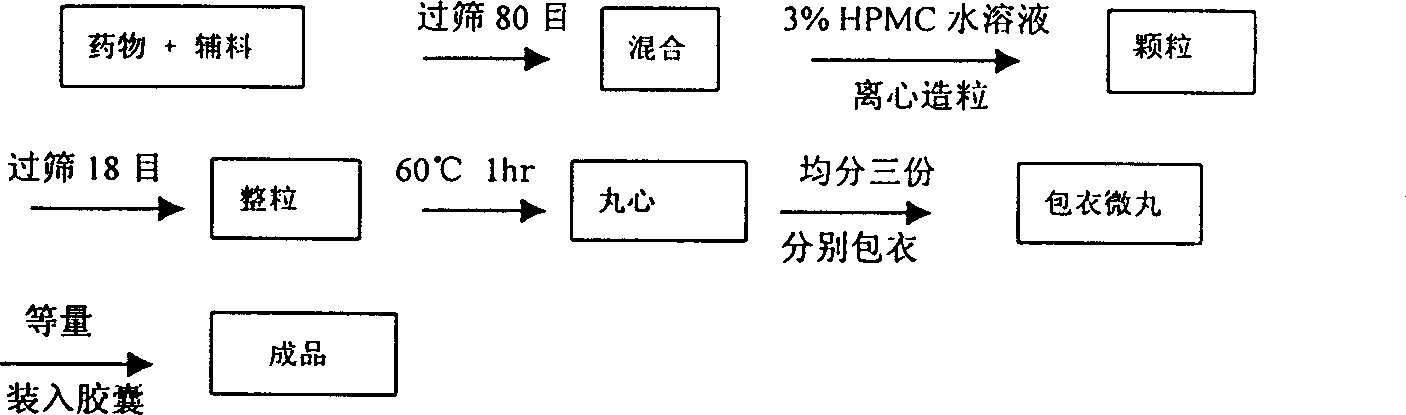
[0016] Preparation of the effective part of Danshen: crush the medicinal material of Danshen into a coarse powder, add 10 times the weight of the medicinal material in water, heat and extract twice at 80°C for 1.5 hours each time, filter, combine the secondary extracts, and rapidly cool to room temperature. According to the ratio of the amount of the upper column sample (equivalent medicinal material) to the amount of wet resin (1:15 / W:V), perform polyamide adsorption column chromatography, and use water at 1-2ml / cm 2 / min flow rate eluted 10 column bed volumes, followed by 60% ethanol 1-2ml / cm 2 10 column bed volumes were eluted at a flow rate of / min, and the eluted part with 60% ethanol was the effective part of the total phenolic acid of salvia miltiorrhiza, and the solvent was recovered under reduced pressure at about 70°C to a liquid extract with a specific gravity of 1.09-1.11. The above liquid extract of salvia miltiorrhiza phenolic acid is dried under reduced pressure...
Embodiment 2
[0018] Preparation of effective part of Panax notoginseng
[0019] Take notoginseng medicinal material and crush it into coarse powder, add 70% ethanol 10 times the weight of the medicinal material, reflux extraction twice, each time for 2.5 hours, filter, combine the second extraction solution, and recover the solvent under reduced pressure at about 70°C until the specific gravity is 1.10 -1.12 liquid extract. Dilute it with water to a concentration of 1:1 (equivalent to medicinal material g / ml), and perform macroporous resin HPD100 adsorption column chromatography according to the ratio of the amount of the column sample (equivalent to medicinal material) to the amount of wet resin (1:15 / W:V), and use water at 1-2ml / cm 2 / min flow rate eluted 10 column bed volumes, followed by 90% ethanol-2ml / cm 2 10 column bed volumes were eluted at a flow rate of / min, followed by 90% ethanol to elute the crude product of Panax notoginseng saponins, reduce the solvent at about 70°C to a ...
Embodiment 3
[0021] Preparation of Bornean Cyclodextrin Inclusion Complex
[0022] Dissolve borneol in ethanol, add 1:15 cyclodextrin, recover the solvent, and dry it.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com