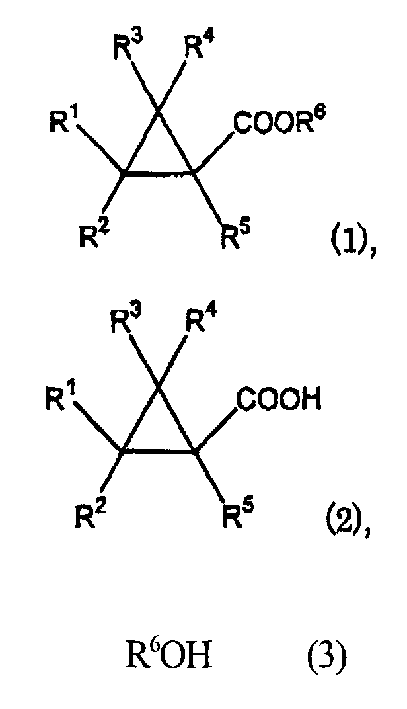
Process for prepn. of cyclopropane formic ether
A technology of cyclopropane formic acid and alkyl, which is applied in the field of preparing cyclopropane formic acid ester, and can solve problems such as ineffective industrial production methods
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0066] Add 0.43 gram of 2,2-dimethyl-3-(2-methyl-1-propenyl) cyclopropanecarboxylic acid (E / Z=80 / 20) in 10 milliliters test tube type reactor, 0.50 gram 3-benzene oxybenzyl alcohol, 5.8 mg of zirconium tetrachloride, and 5 mL of xylene. The reactor was equipped with a Dean-Stark trap and a condenser, and the reaction mixture was stirred at 145°C under reflux for 8 hours while separating and collecting the water produced in the reaction as a by-product in the trap . The reaction mixture thereof was analyzed by gas chromatography, and it was found that 2,2-dimethyl-3-(2-methyl- (3-phenoxyphenyl)methyl 1-propenyl)cyclopropanecarboxylate.
Embodiment 2
[0068] A reaction was carried out in a manner similar to that of Example 1, except that 9.4 mg of a complex of zirconium tetrachloride and 2-tetrahydrofuran was added instead of 5.8 mg of zirconium tetrachloride in Example 1.
[0069] The reaction mixture thereof was analyzed by gas chromatography, and it was found that 2,2-dimethyl-3-(2-methyl- (3-phenoxyphenyl)methyl 1-propenyl)cyclopropanecarboxylate.
Embodiment 3
[0071] The reaction was carried out in a similar manner to Example 1, except that 7.3 mg of zirconocene dichloride was added instead of 5.8 mg of zirconium tetrachloride in Example 1.
[0072] The reaction mixture thereof was analyzed by gas chromatography, and it was found that 2,2-dimethyl-3-(2-methyl-1-propenyl)cyclopropanecarboxylic acid was obtained in a yield of 97% (selectivity: 98%) based on alcohol species (3-phenoxyphenyl)methyl ester.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com