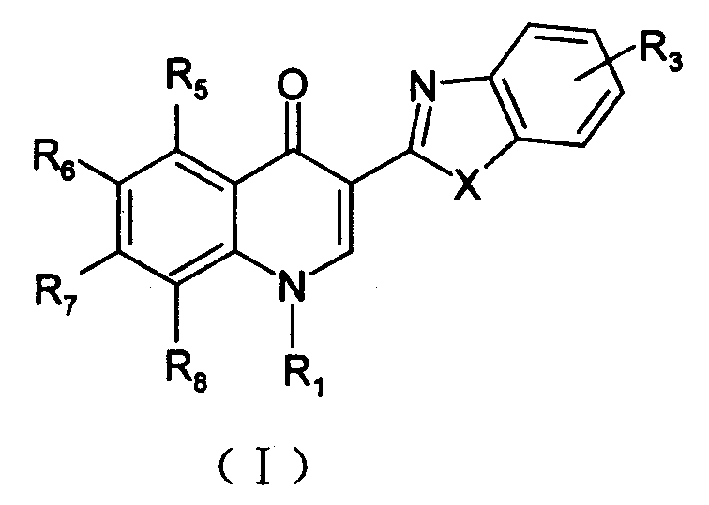
3-position substituted quinolone derivativers and its use in pharmacy
A representative compound technology, applied in the 3-position substituted quinolone derivatives and its application in medicine, can solve the problem of unreported anti-tumor activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] 1-Ethyl-3-(2-benzoimidazolyl)-6-fluoro-7-(1-piperazinyl)-4(1H)-quinolone (1)
[0036] Norfloxacin (norfloxacin) (3.19 grams, 0.1mol) and o-phenylenediamine (1.08 grams, 0.1mol) were mixed and ground in a mortar, added to the reaction bottle, 40 milliliters of polyphosphoric acid was added, and vacuum pumping Remove the air in the reaction bottle until no bubbles are generated, feed nitrogen, stir slowly to 140°C and keep warm until the reaction solution is homogeneous, then raise the temperature to 180°C and react for 4 hours, then cool down to about 100°C, and stir the reaction solution down. Put it into 250 grams of crushed ice, let it stand for cooling, adjust the pH value to 9, suction filter, and dry to obtain the crude product, DMF recrystallization, and methanol-triethylamine as the developer column chromatography to obtain 0.90 grams of off-white crystals, mp 260-2°C, yield 23.4%. IR (cm -1 ): 3923, 2947, 1630, 1563, 1542, 1527, 1492, 1255, 879; 1 HNMR (δ, pp...
Embodiment 2
[0038] 1-Ethyl-3-(2-benzoimidazolyl)-6-fluoro-7-(4-methyl-1-piperazinyl)-4(1H)-quinolone (2)
[0039] Using flufloxacin as a raw material, the preparation method is the same as in Example 1, and recrystallized with DMF. Column chromatography gave off-white solid, mp239-42°C (decomposition), yield 26.4%. IR (cm-1): 3329, 2925, 1620, 1550, 1473, 1284, 789; 1HNMR (δ, ppm, DMSO-d6): 1.44(t, 3H, -CH 3 ), 2.25(s, 3H, -CH 3 ), 2.50 (m, 4H, -CH 2 -*2), 3.27(m, 4H, -CH 2 -*2), 4.56(q, 2H, -CH 2 -), 7.12(m, 3H, 8-H and 5'-Hand 6'-H), 7.61(m, 2H, 4'-H and 7'-H), 7.95(d, 1H, 5-H) , 9.17(s, 1H, 1-H), 12.67(s, 1H, 1'-H); Formula: C 23 h 24 N 5 OF.2H 2O MW: 441.50, Anal (C%, H%, N%,) Calc: 62.57, 6.34, 15.86 Found: 62.02, 6.13, 16.26; HRMS: M + Meas 405.197192 Calc mass 405196474 Error-0.718, MS (EI, m / s): (M + ): 405 (base peak), 306
Embodiment 3
[0041] (±) 6-(2-Benzimidazolyl)-9-fluoro-2,3-dihydro-3-methyl-10-(4-methyl-1-piperazinyl)-7-oxo-7H -pyrido[1,2,3-de][1,4]benzoxazine (3)
[0042] Using ofloxacin as a raw material, the preparation method is the same as in Example 1, and recrystallized with DMF. Column chromatography gave an off-white solid, mp230-2°C, yield 21.3%.
[0043] IR (cm-1): 3332, 2930, 1620, 1575, 1550, 1473, 1285, 1259, 789; 1HNMR (δ, ppm, DMSO-d6): 1.48 (d, 3H, -CH 3 ), 2.32 (s, 3H, -CH 3 ), 2.51(s, 4H, -CH 2 -*2), 3.33(s, 4H, -CH 2 -*2), 4.39-4.59(m, 2H, -CH 2 -), 4.92(m, 1H, -CH-), 7.14(m, 2H, 5'-Hand 6'-H), 7.60(d, 3H, 4'-H, 7'-H and8-H), 9.15(sH, 5-H), 12.67(s, 1H, 1'-H); Formula: C 24 h 24 N 5 o 2 F.1.5H 2 O MW: 460.51, Anal (C%, H%, N%,) Calc: 62.60 5.90, 15.21 Found: 62.94, 5.46, 15.00; HRMS: M+Meas 433.191533 Calc mass 433.191388 Error-0.145mu, MS (EI, m / s): 433 (M+base peak).
PUM
| Property | Measurement | Unit |
|---|---|---|
| Yield | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com