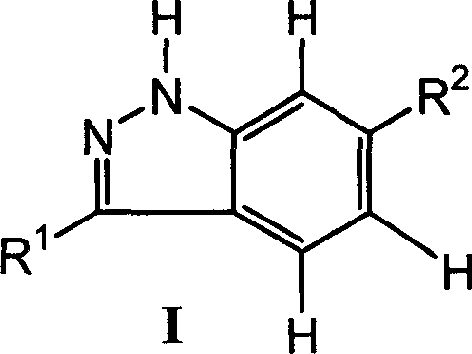
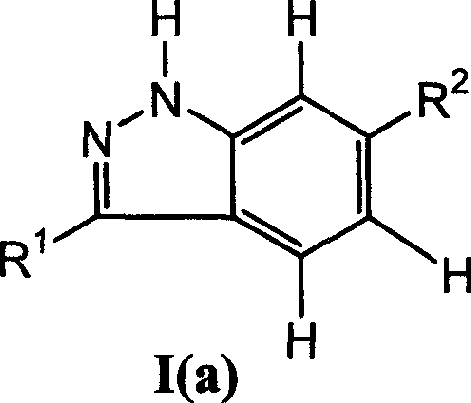
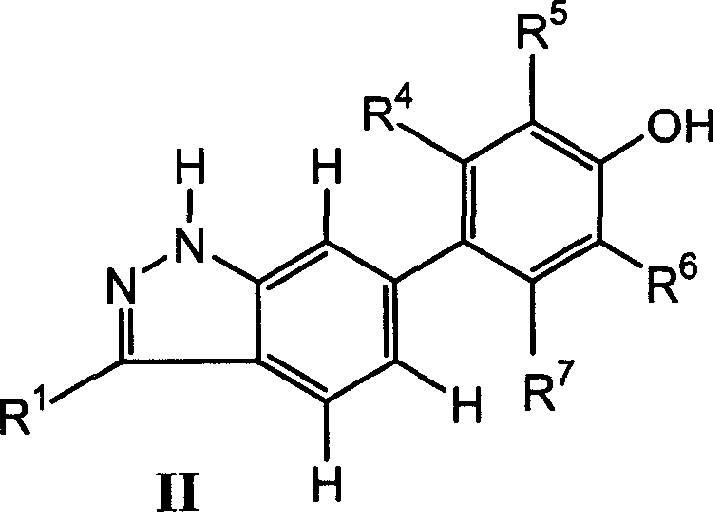
Indazol compound for inhibiting protein kinase and medicine composition and their application
A technology of compounds and prodrugs, applied in the field of diaminothiazole compounds
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0147] Example 1(a): 3-[E-2-(3,4-dimethoxy-phenyl)vinyl]-6-(3-methoxy-4-hydroxy-phenyl)-1H- Indazole
[0148]
[0149]3-[E / Z-2-(3,4-dimethoxy-phenyl)vinyl]-6-[3-methoxy-4-(methoxymethoxy)phenyl]- 1H-Indazole (-205 mg, 0.461 mmol (theoretical)) was dissolved in tetrahydrofuran (THF, 10 ml) and then treated with water (10 ml) and trifluoroacetic acid (TFA, 20 ml). The reaction mixture was stirred at 23°C for 30 minutes. The mixture was diluted with toluene and the volatiles were removed under reduced pressure (30 mmHg, 35°C) to give a concentrated volume of ~5ml. Additional toluene (100ml) was then added and the mixture concentrated under reduced pressure to give crude material still containing some acid. The material was partitioned between ethyl acetate and saturated sodium bicarbonate, the organic material was separated, dried over sodium sulfate, decanted and concentrated under reduced pressure. The residue, the isomer of the olefin (-185 mg, 0.461 mmol (theoretical))...
Embodiment 2
[0177] Example 2(a): 3-(naphthalen-2-yl)-6-(3-methoxy-4-hydroxy-phenyl)-1H-indazole
[0178]
[0179]Dissolve 6-(4-benzyloxy-3-methoxy-phenyl)-3-naphthalen-2-yl-1H-indazole (25mg, 0.055mmol) in ethyl acetate (2ml), benzene (2ml ) and methanol (2ml). Palladium-coated activated carbon (25 mg, 10% wt) was added to the solution, and the reaction vessel was pumped / blown with hydrogen for 5 cycles. The reaction mixture was stirred at 23°C for 3 days and filtered through celite. Concentration and purification by silica gel chromatography afforded 3-(naphthalen-2-yl)-6-(3-methoxy-4-hydroxy-phenyl)-1H-indazole (8 mg, 40%): 1 H NMR (CDCl 3 )δ10.3(bs, 1H), 8.50(s, 1H), 8.20(d, 1H, J=8Hz), 7.98(d, 1H, J=8Hz), 7.90(m, 1H), 7.7-6.8( m, 9H), 3.98 (s, 3H). MS(ES) [M+H] / z Calcd 367, Found 367, [m-H] / z Calc'd 365, Found 365.
[0180] The starting materials were prepared as follows:
[0181] (i)
[0182]
[0183] 2-Bromnaphthalene (117mg, 0.564mmol, 6.0eq) was dissolved in THF (0.75...
Embodiment 3
[0200] Example 3: 3-(1H-indol-2-yl)-6-(,3-methoxy-4-hydroxy-phenyl)-1H-indazole
[0201]
[0202] According to the method described in Example 1 (a), 3-(1H-benzimidazol-2-yl)-6-(3-methoxy-4-methoxymethoxy-phenyl)-1H- Indazole was converted to 4-[3-(1H-benzimidazol-2-yl)-1H-indazol-6-yl]-2-methoxyphenol (5.3 mg, 28%). HRMS (FAB) [m+H] / z calcd. 357.1351, found 357.1349.
[0203] The starting materials were prepared as follows:
[0204]
[0205] 6-(3-Methoxy-4-methoxymethoxy-phenyl)-1H-indazole-3-carbaldehyde (step (vi) of Example 1(a)) (20 mg, 0.064 mmol, 1 equiv) was dissolved in degassed 1:1 MeOH-water (0.7ml) and treated with acetic acid (19 μl, 5 equiv), 1,2-phenylenediamine (8.3 mg, 1.2 equiv) and ketone acetate (II ) (18 mg, 1.4 equivalents) treatment. The mixture was stirred for 30 minutes, diluted with ethanol (3ml) and water (2ml), washed with SH 2 Air flow was bubbled for 3 minutes to obtain a black precipitate. The mixture was stirred for 12 hours. The mi...
PUM
| Property | Measurement | Unit |
|---|---|---|
| mass | aaaaa | aaaaa |
| purity | aaaaa | aaaaa |
| composition ratio | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com