Fluorescent brightening agent and synthesis method of its mixture
The technology of a fluorescent whitening agent and a synthesis method is applied in the field of synthesis of fluorescent whitening agents and their mixtures, which can solve problems such as harsh reaction conditions, high production costs, and low product purity, and achieve safe reaction processes, reduced raw material costs, Good whitening effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
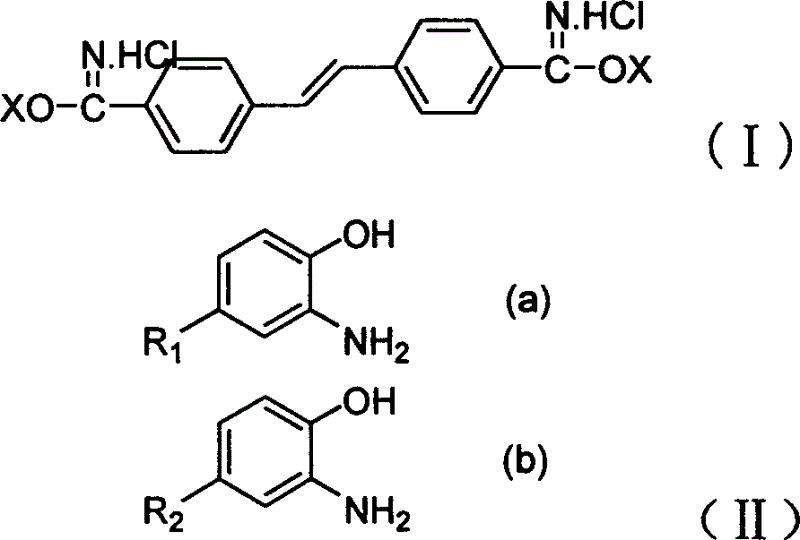
[0028] 4,4'dicyano-stilbene is prepared according to the synthetic method in US 4519953;
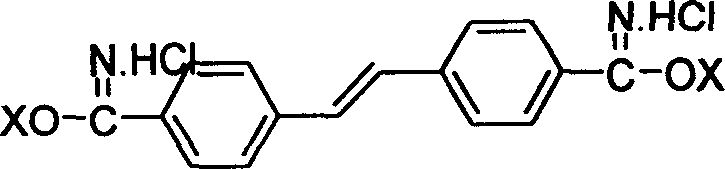
[0029] Disperse 7.1g (0.031 mol) of 4,4'-dicyano-stilbene in 200ml of methanol, pass through dry hydrogen chloride gas to saturation, stir and react at room temperature for 50 hours, filter, and dry in vacuo to obtain stilbene - 11.0 g of 4,4'-bisiminomethyl ester hydrochloride.
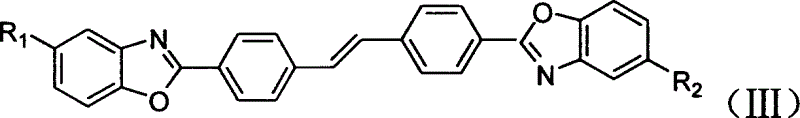
[0030] 11.0 g (0.03 mol) of stilbene-4,4'-bisiminomethyl ester hydrochloride and 6.7 g (0.061 mol) of 2-aminophenol were added to 60 ml of methanol, followed by 40 ml of glacial acetic acid. Slowly heat up to about 70°C with stirring, and keep for 6 hours until the end of the reaction. Cool down to 40°C, vacuum filter, wash the filter cake with water and then dry it. 11.4 g of 4,4'-bis(benzoxazol-2-yl)stilbene was obtained.
example 2
[0032] 11.0g (0.03mol) stilbene-4,4'-bisimine methyl ester hydrochloride, 3.4g (0.031mol) 2-aminophenol, 3.9g (0.031mol) 4-methyl-2-amino Phenol was added to 60ml of methanol, followed by 40ml of glacial acetic acid. Slowly heat up to about 70°C with stirring, and keep for 6 hours until the end of the reaction. Cool down to 40°C, vacuum filter, wash the filter cake with water and then dry it. 11.9 g of the mixture were obtained.
[0033] The percentage of the mixture determined by liquid chromatography is:
[0034] 4-(Benzoxazol-2-yl)-4'-(5-methylbenzoxazol-2-yl)stilbene 49.5%; 4,4'-bis(benzoxazol-2- base) stilbene 22.7%; 4,4'-bis(5-methylbenzoxazol-2-yl) stilbene 27.8%.
example 3
[0036] 11.0g (0.03mol) stilbene-4,4'-bisiminomethyl ester hydrochloride, 2.6g (0.024mol) 4-methyl-2-aminophenol and 6.9g (0.042mol) 4-tert Butyl-2-aminophenol was added to 60ml of methanol, followed by 40ml of glacial acetic acid. Slowly heat up to about 70°C with stirring, and keep for 6 hours until the end of the reaction. Cool down to 40°C for vacuum suction filtration, wash the filter cake with water and then dry it. 13.7 g of the mixture were obtained.
[0037] Determine the percentage composition of the mixture with liquid chromatography as:
[0038] 4-(5-methylbenzoxazol-2-yl)-4'-(5-tert-butylbenzoxazol-2-yl)stilbene 44.0%; 4,4'-bis(5- Tolylbenzoxazol-2-yl)-stilbene 8.2%; 4,4'-bis(5-tert-butylbenzoxazol-2-yl)-stilbene 47.8%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com