Medicine composition containing taxol substance and its preparation method
A composition, paclitaxel technology, applied in the direction of drug combination, antineoplastic drugs, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0048] Example 1: Preparation of a reconstitutable, solid paclitaxel / acetyl-γ-cyclodextrin composition by dry method:
[0049] Dissolve 500mg of acetyl-γ-cyclodextrin, 3mg of citric acid, and 1.5mg of sodium citrate in 2.5ml of water, add 3mg of paclitaxel (or first dissolve paclitaxel in 0.25ml of ethanol), stir until a clear solution is obtained, and freeze-dry to obtain solid matter.
[0050] 100 mg of this solid was easily dissolved in 1 ml of 0.9% NaCl (or 5% G) aqueous solution to give clear solutions with a paclitaxel content of 0.99 mg / ml, respectively. The reconstituted solution was stored in a sealed glass container at 35° C. for 6 hours, and the HPLC analysis results are shown in Table 8.
[0051] Paclitaxel final concentration: 0.99mg / ml
Embodiment 2
[0052] Example 2: Preparation of a reconstitutable, solid paclitaxel / acetyl-γ-cyclodextrin composition by dry method:
[0053] Dissolve 500 mg of acetyl-γ-cyclodextrin and 3 mg of tartaric acid in 2.5 ml of water, add 3 mg of paclitaxel (or first dissolve paclitaxel in 0.25 ml of ethanol), stir until a clear solution is obtained, and freeze-dry to obtain a solid substance.
[0054] 100 mg of this solid was readily dissolved in 2 ml of 0.9% NaCl (or 5% G) aqueous solution to give clear solutions with a paclitaxel content of 0.49 mg / ml, respectively. The reconstituted solution was stored in a sealed glass container at 35° C. for 6 hours, and the HPLC analysis results are shown in Table 9.
[0055] Paclitaxel final concentration: 0.49mg / ml
Embodiment 3
[0056] Example 3: Preparation of reconstitutable, solid Docetaxel / HP-β-CD compositions by dry method:
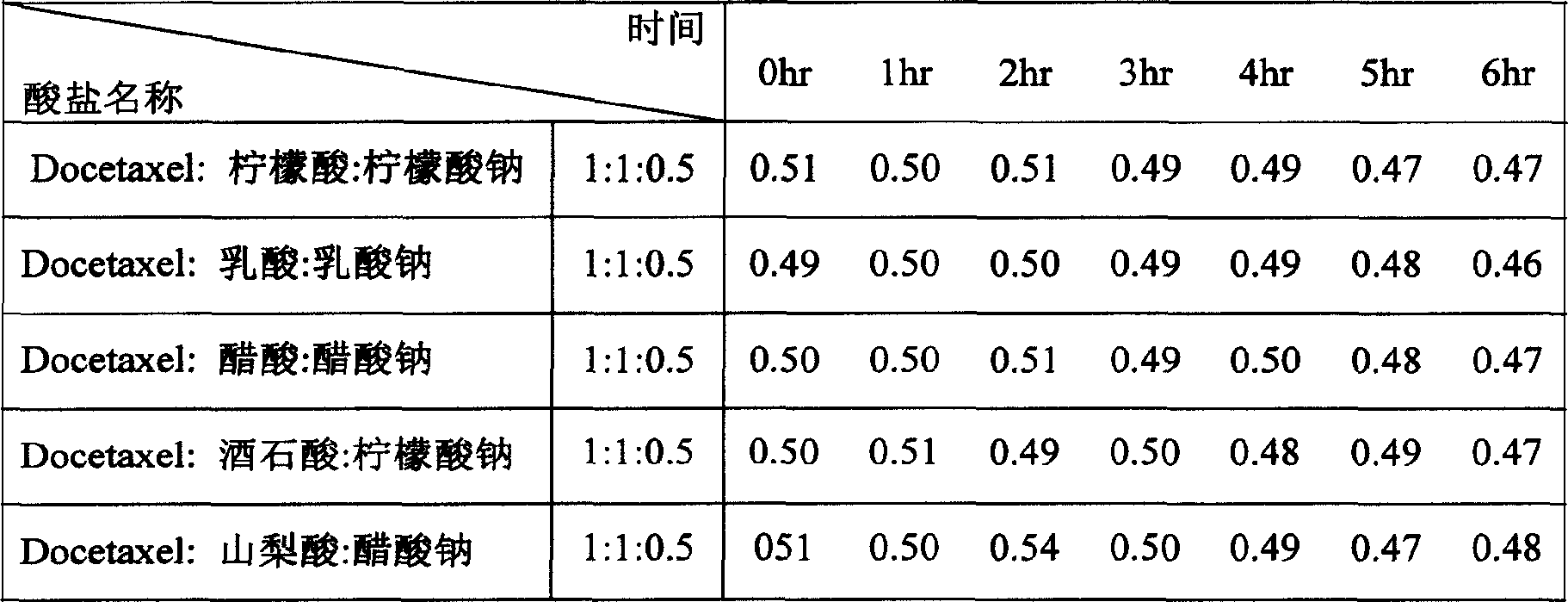
[0057] Dissolve 420mg of HP-β-CD, 6mg of lactic acid, and 2mg of sodium lactate in 2.5ml of water, add 6mg of Docetaxel (or first dissolve Docetaxel in 0.15ml of ethanol), stir until a clear solution is obtained, and freeze-dry to obtain a solid substance.
[0058] 70 mg of this solid was readily dissolved in 1 ml of 0.9% sodium chloride (or 5% dextrose) aqueous solution to give clear solutions with a Docetaxel content of 1.00 mg / ml, respectively. The reconstituted solution was stored in a sealed glass container at 35° C. for 6 hours, and the HPLC analysis results are shown in Table 10.
[0059] Docetaxl final concentration: 1.00mg / ml
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com