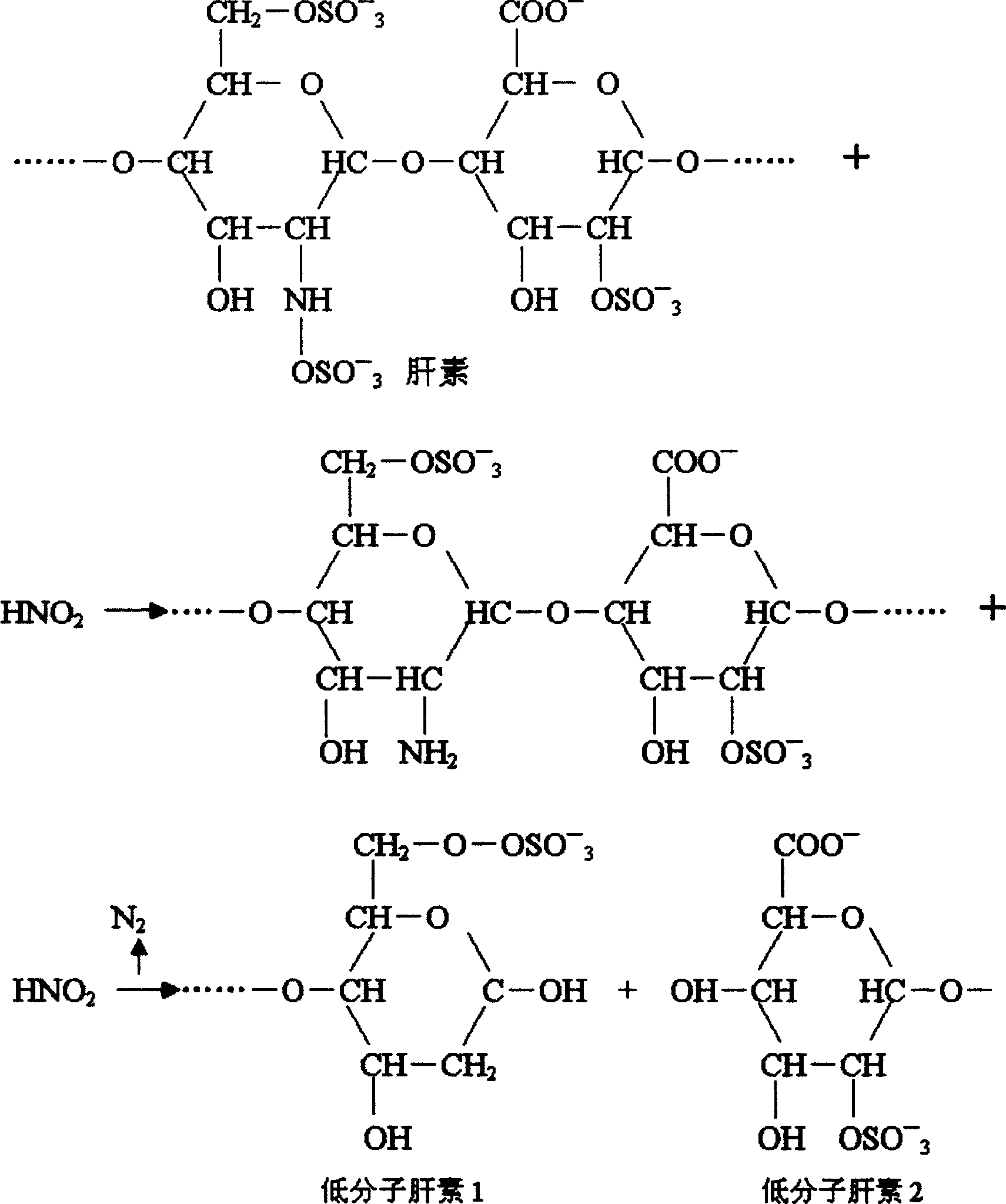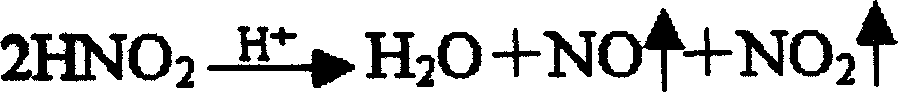Process for preparing low moledule heparin calcium of low nitrite content
A low-molecular-weight heparin calcium and low-nitrite technology, which is applied in the directions of medical preparations containing active ingredients, pharmaceutical formulations, organic active ingredients, etc., can solve the problems of complex process, many steps and large quantities of low-molecular-weight heparin calcium, etc. To achieve the effect of simple process, convenient operation and low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Dissolve 800 grams of refined heparin sodium in 20 liters of acetic acid aqueous solution at pH 3.0, add 20 grams of sodium nitrite to the solution, and crack at 25°C for 4 hours to obtain a cracking solution; neutralize the solution with calcium hydroxide to obtain pH 5 .5 solution; filter, discard the insoluble matter, the filtrate precipitates with saturated calcium chloride ethanol solution, collects the precipitate and dissolves it in water for injection, adjusts the pH of the solution with hydrochloric acid, and obtains a solution of pH 0.3; Neutralize the solution to obtain a solution with a pH of 6.0; filter, discard the insoluble matter, precipitate the filtrate with a saturated calcium chloride ethanol solution, collect the precipitate, and dry it under reduced pressure to obtain the finished low-molecular-weight heparin calcium.
Embodiment 2
[0035] Dissolve 800 grams of refined heparin sodium in 20 liters of acetic acid aqueous solution of pH 3.4, add 28 grams of sodium nitrite to the solution, and crack at 27 ° C for 4.5 hours to obtain a lysis solution; neutralize the solution with calcium hydroxide to obtain a pH 6 .0 solution; filter, discard the insolubles, precipitate the filtrate with saturated calcium chloride ethanol solution, collect the precipitate and dissolve it in water for injection, adjust the pH of the solution with hydrochloric acid to obtain a solution of pH 1.0; use calcium hydroxide Neutralize the solution to obtain a solution with a pH of 6.5; filter, discard the insoluble matter, precipitate the filtrate with a saturated calcium chloride ethanol solution, collect the precipitate, and dry it under reduced pressure to obtain the finished low-molecular-weight heparin calcium.
Embodiment 3
[0037] Dissolve 800 grams of refined heparin sodium in 22 liters of acetic acid aqueous solution at pH 3.2, add 28 grams of sodium nitrite to the solution, and crack at 23°C for 5 hours to obtain a lysis solution; neutralize the solution with calcium hydroxide to obtain pH 5 .8 solution; filter, discard the insoluble matter, and the filtrate is precipitated with saturated calcium chloride ethanol solution, collects the precipitate and dissolves it in water for injection, adjusts the pH of the solution with hydrochloric acid, and obtains a solution of pH 0.5; Neutralize the solution to obtain a solution with a pH of 6.2; filter, discard the insoluble matter, precipitate the filtrate with a saturated calcium chloride ethanol solution, collect the precipitate, and dry it under reduced pressure to obtain the finished low-molecular-weight heparin calcium.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com