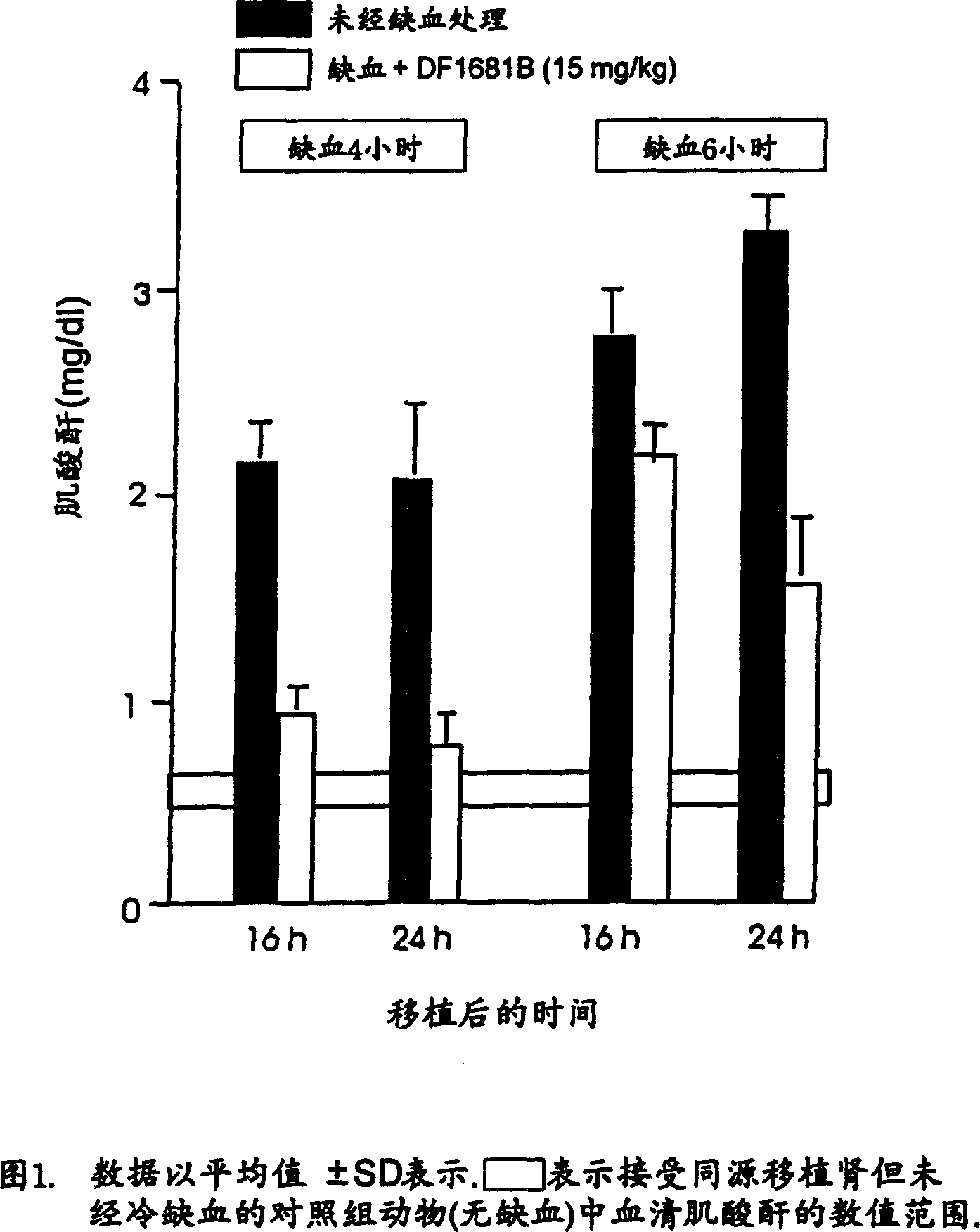
Use of (R)-ibuprofen methanesulfonamide and salts thereof in the treatment and prevention of rejection reactions of transplanted organs
A technology for isobutyl phenylpropionate methylsulfonamide and organ transplantation, applied in the application field of (R)- ibuprofen methylsulfonamide and its salt in the treatment and prevention of transplanted organ rejection, capable of Address issues such as increased risk of acute rejection
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Preparation of (R)-isobutylphenylpropionic acid methylsulfonamide
[0031] The suspension of R(-)-2-(4-isobutylphenyl)-propionic acid (R-isobutylphenylpropionic acid, 4g, 0.019mol) and thionyl chloride (7.4mL) was refluxed for 4 hours; Cool naturally at room temperature. Excess thionyl chloride was evaporated in vacuo.
[0032] The residue was washed twice with a few drops of anhydrous dioxane, and the solvent was evaporated in vacuo to remove the last remaining thionyl chloride. 4.66 g (0.019 mol) of a yellow oil, R(-)-2-(4-isobutylphenyl)-propionyl chloride, were obtained, which was dissolved in a few ml of anhydrous tetrahydrofuran (THF).
[0033] In addition, methylsulfonamide (2.3 g, 0.0243 mol) was added to a suspension of potassium tert-butoxide (2.73 g, 0.0244 mol) and anhydrous tetrahydrofuran (28 mL), and the mixture was stirred at room temperature for 30 minutes. Then, R(-)-2-(4-isobutylphenyl)-propionyl chloride (4.66 g, 0.019 mol) obtained above was adde...
Embodiment 2
[0036] Preparation of L(+)-Lysine Salt (DF 1681B) of (R)-Isobutylphenylpropionic Acid Methylsulfonamide
[0037] A solution of L(+)-lysine (129 mg, 0.88 mmol) in water (1.3 mL) was added to R(-)-N-[2-(4-isobutylphenyl)propionyl]-methylsulfonyl A solution of the amide (250 mg, 0.88 mmol) in 1 mL of methanol. The solvent was evaporated and the residue was dissolved in ether (5 mL) and stirred at room temperature overnight. The separated highly hygroscopic crystalline substance was quickly filtered under a nitrogen atmosphere, washed with anhydrous ether on the filter membrane, and vacuum-dried at 50°C for 2 hours to obtain 360 mg of R(-)-N-[2-(4-iso Butylphenyl)propionyl]-methanesulfonamide L(+)-lysine salt, which is a pale yellow powder. [α] D =-17.3 (c=1.15; methanol); 1 H-NMR (D 2 O): δ7.30(dd, 4H, J=8Hz); 3.77(t, 1H, J=7Hz); 3.65(q, 1H, J=7Hz); 3.05(m, 5H); 2.52(d, 2H , J=7Hz); 1.92(m, 2H); 1.75(m, 2H); 1.50(m, 3H), 1.40(d, 3H, J=7Hz), 0.90(6H, d, J=7Hz).
[0038] ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com