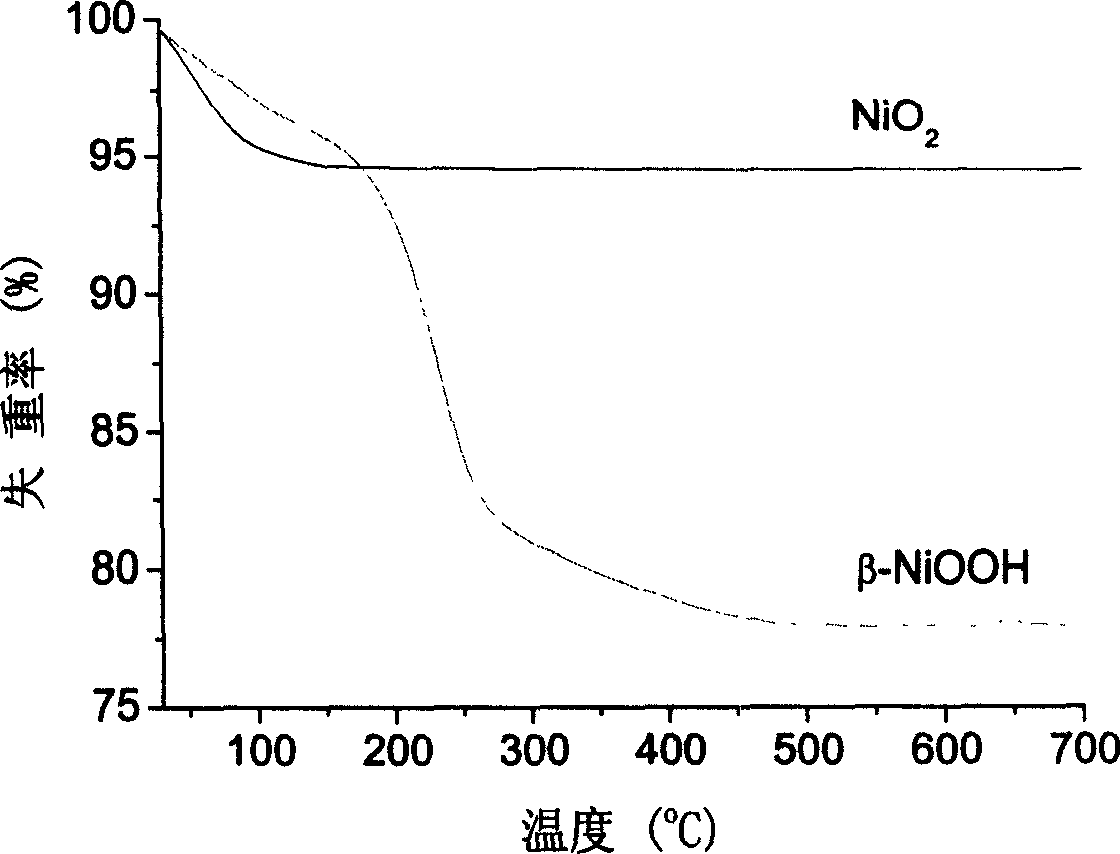
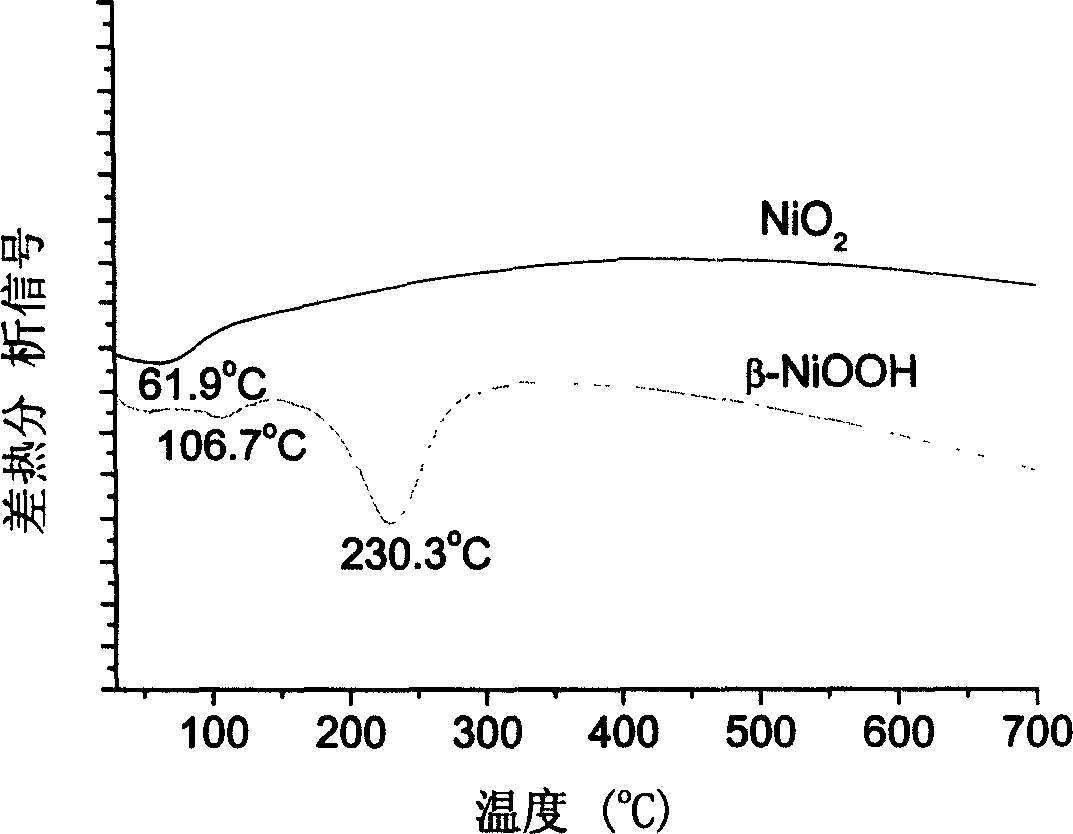
Preparation method of nano-nickel dioxide
A technology of nickel dioxide and nanometer, which is applied in the field of preparation of nanocatalytic materials, can solve the problems of poor thermal stability, particle diameter not in the nanometer range, increase the post-processing process, etc., and achieve the effect of reducing liquid discharge and fine nanostructure
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0013] Weigh 4g of sodium carbonate and 9g of sodium hydroxide and dissolve in 120mL of water to form a mixed alkali solution, and dissolve 14g of nickel nitrate hexahydrate in 60mL of water. While stirring, slowly drop nickel nitrate into the mixed alkali. After dropping, continue stirring at 50°C for 20h. The precipitate was then washed until the filtrate was neutral. It is then dried at 90°C, calcined at 350°C, and ground to obtain black nickel dioxide with a particle diameter of 10-40nm.
Embodiment 2
[0015] Weigh 8g of sodium carbonate and 7g of sodium hydroxide and dissolve in 120mL of water to form a mixed alkali solution, and dissolve 18g of nickel nitrate hexahydrate in 60mL of water. While stirring, slowly drop nickel nitrate into the mixed alkali. After dropping, continue stirring at 70°C for 15h. The precipitate was then washed until the filtrate was neutral. It is then dried at 120°C, calcined at 500°C, and ground to obtain black nickel dioxide with a particle diameter of 20-40nm.
Embodiment 3
[0017] Weigh 6g of sodium carbonate and 8g of sodium hydroxide and dissolve in 120mL of water to form a mixed alkali solution, and take 19g of nickel nitrate hexahydrate and dissolve in 60mL of water. While stirring, slowly drop nickel nitrate into the mixed alkali. After dropping, continue stirring at 80°C for 10h. The precipitate was then washed until the filtrate was neutral. It is then dried at 100°C, calcined at 450°C, and ground to obtain black nickel dioxide with a particle diameter of 10-40nm.
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com