Human source anti-hepatitis A virus gene engineering antibody from CHO cell
A technology of genetic engineering antibody and hepatitis A virus, which is applied in the direction of genetic engineering, antiviral immunoglobulin, antibody, etc., and can solve problems such as immune diseases and antibody failure
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
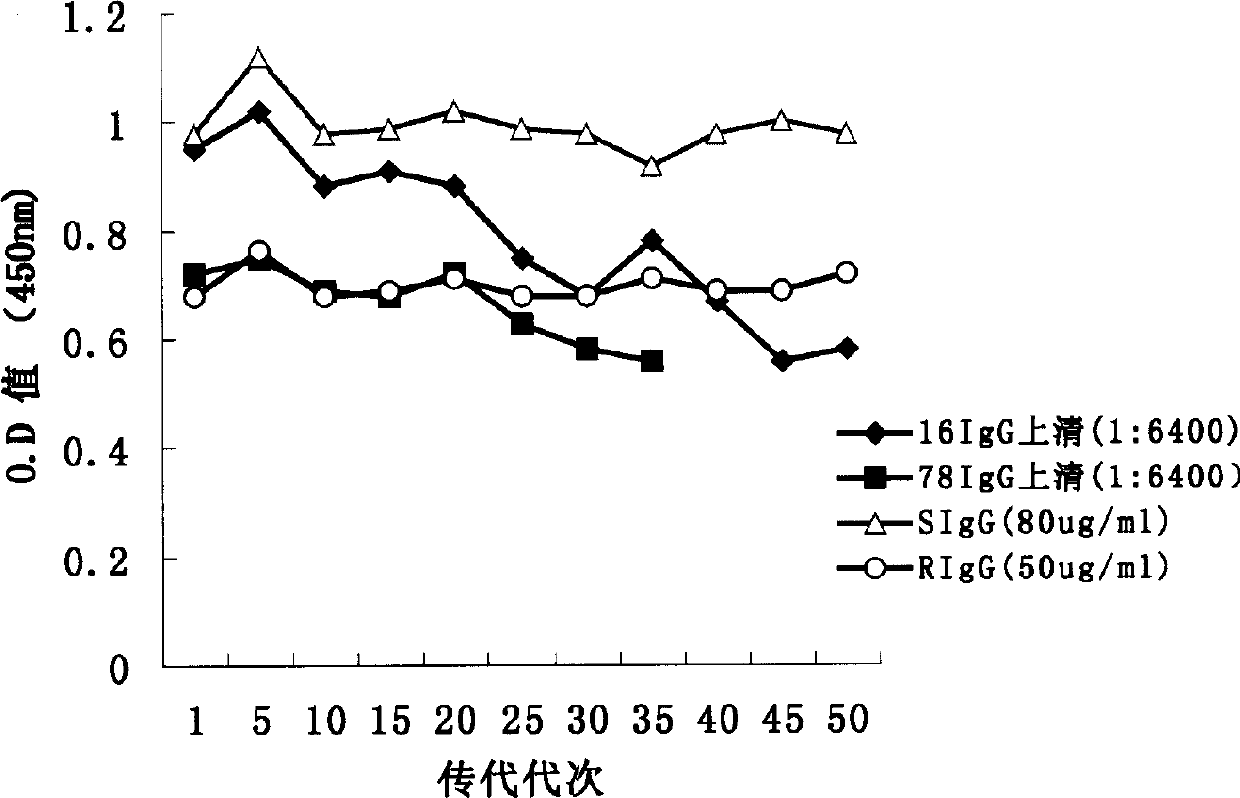
Image
Examples
Embodiment 1
[0034]Embodiment 1 is the sequence characteristics of the human source anti-hepatitis A virus genetic engineering antibody gene produced by CHO cells; embodiment 2-4 is the cloning preparation method of the human source anti hepatitis A virus genetic engineering antibody produced by CHO cells; embodiment 5 is CHO cell The protein characteristics of the produced human-sourced anti-hepatitis A virus genetically engineered antibody; Examples 6-9 are the functional characteristics of the human-sourced anti-hepatitis A virus genetically engineered antibody produced by CHO cells.
example 1
[0035] Example 1, the sequence characteristics of the human anti-hepatitis A virus genetically engineered antibody gene produced by CHO cells: its variable region gene composition characteristics are specific light chain and heavy chain genes, derived from the human anti-hepatitis A virus antibody gene library Specific enrichment screening and expression, the corresponding three CDR region sequences are novel sequences unique to this antibody, and the ChHAIgG16 light chain and heavy chain variable region gene sequences have been stated in the patent application CN1316437A. ChHAIgG78 light chain variable region CDR1, CDR2 and CDR3 sequences are "RASQSVSSSYLA", "GASSRAT" and "QQYGSSPLT", respectively. Its complete nucleotide sequence is as follows:
[0036] GAGCTCACGCAGTCTCCA GGCACCCTGTCTTTGTCTCCAGG GAAAGAGCC
[0037] ACC CTC TCC TGC AGG GCCAGTCAGGTGT AGCAGCAGCTACTTA GCC
[0038] TGGTACCAGCAGAAACCTGGCCAGGCTCCCAGGCTCCTCATCTATGGTGCATC
[0039] CAGCAGGGCCACTGGC ATCCCAGACAGGTTCAG...
example 2
[0052] Example 2, Cloning and recombination of the human anti-hepatitis A virus genetically engineered antibody gene produced by CHO cells: the anti-hepatitis A virus neutralizing antibody Fab gene obtained through the phage surface display system was cloned into the human antibody high-efficiency expression expression vector invented by our laboratory middle. Among them, the ChHAIgG16 and ChHAIgG78 light chain variable region genes were respectively cloned into the vector VK-dhfrl with the EcoRV and XhoI endonuclease sites (this vector has been stated in the patent application with the patent publication number of CN1363682), placed in the EF-1α promoter Under the control of the child, the expression vectors 16VL-dhfr and 78VK-dhfr were obtained. The heavy chain variable region genes of ChHAIgG16 and ChHAIgG78 were respectively cloned into the vector VH-dhfr1 with BssHII and BstE II (this vector has been stated in the patent application CN1363682), and the expression vectors ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com