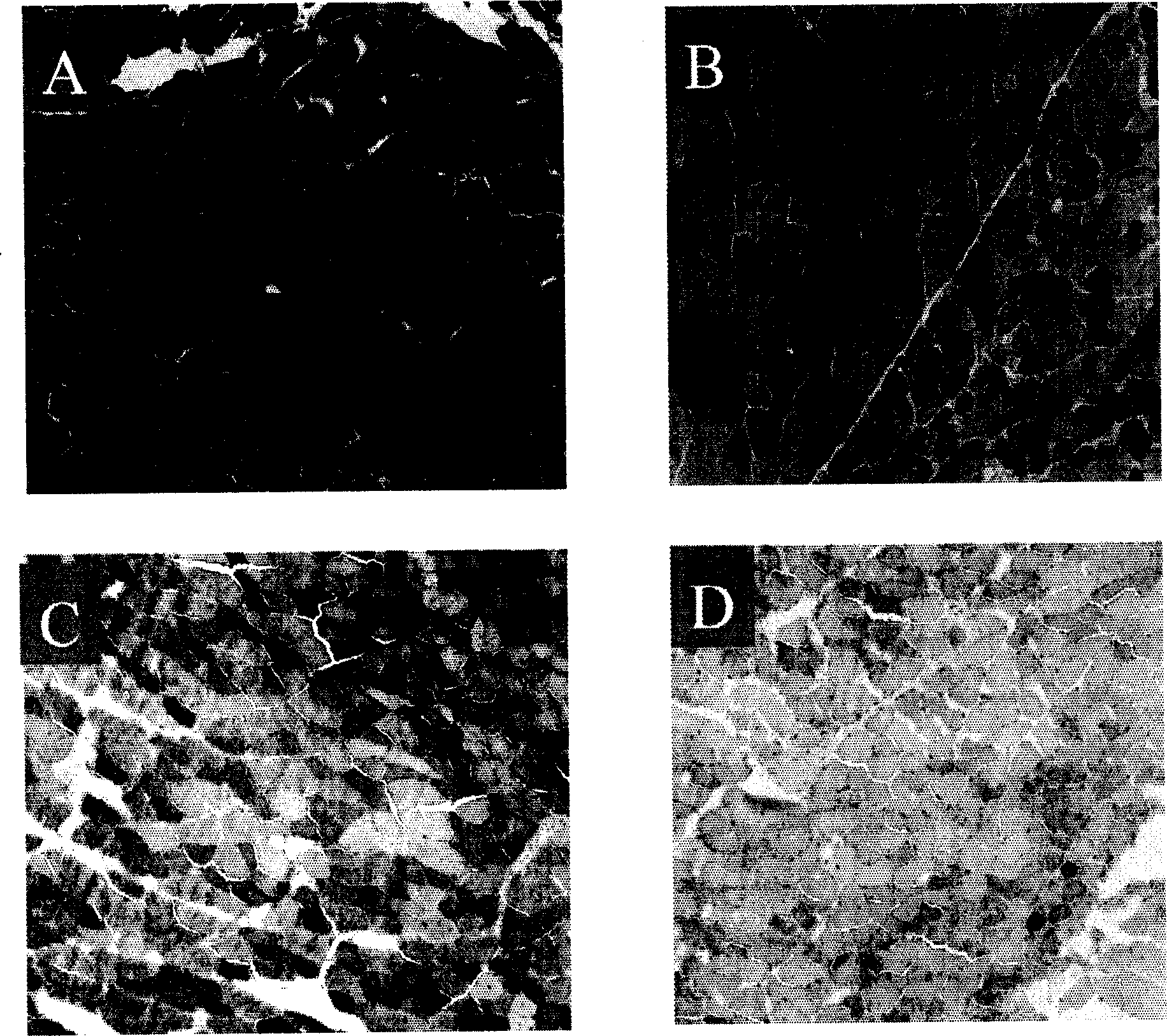
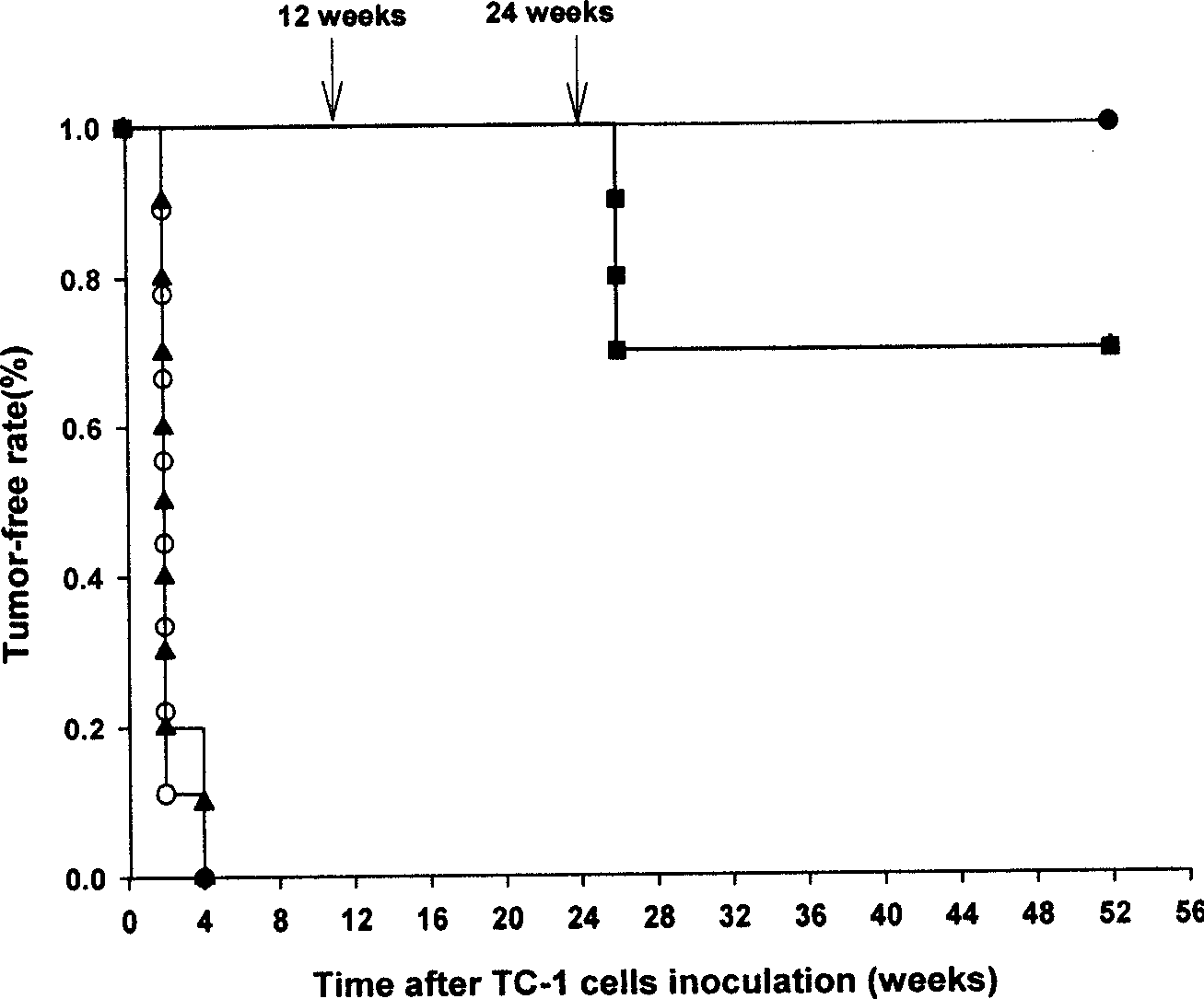
Gene engineering recombined vaccine of carcinoma of cervix uterus
A cervical cancer and genetic engineering technology, which is applied in the field of recombinant vaccines for the treatment and prevention of cervical cancer, can solve the problems such as the need for further confirmation of the immune effect of the vaccine and the problem of carcinogenesis.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0017] The following describes in detail with reference to the accompanying drawings and the related concepts and methods of the present invention.
[0018] 1. Relevant characteristics of recombinant adeno-associated virus:
[0019] Adeno-associated virus (AAV) is a single-stranded virus that has been extensively studied as a vector for gene therapy. As a defective human parvovirus, AAV possesses many natural attributes that make it a vector for human gene therapy, such as non-pathogenicity, site-directed integration capability, and a broad host range (human, simian, murine, canine, and avian). )Wait. Unlike other viral vectors (such as vaccinia virus or adenovirus, etc.) that have been used in vaccines, AAV vectors do not express any viral genes. The only viral DNA that must be included in an AAV vector is its 145 bp inverted terminal repeat. The expressed gene carried by the AAV vector expresses only the cloned gene itself.
[0020] Previous reports have demonstrated tha...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com