Crosslinkable aromatic resin having protonic acid group, and ion conductive polymer membrane, binder and fuel cell using the resin
An aromatic and cross-linking technology, applied in solid electrolyte fuel cells, fuel cells, fuel cell components, etc., can solve problems such as deterioration of battery characteristics, poor power generation efficiency, low heat resistance and chemical durability , to achieve excellent solvent solubility and thermoplasticity, excellent electricity generation efficiency and reliability, and excellent adhesiveness
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
specific example 1
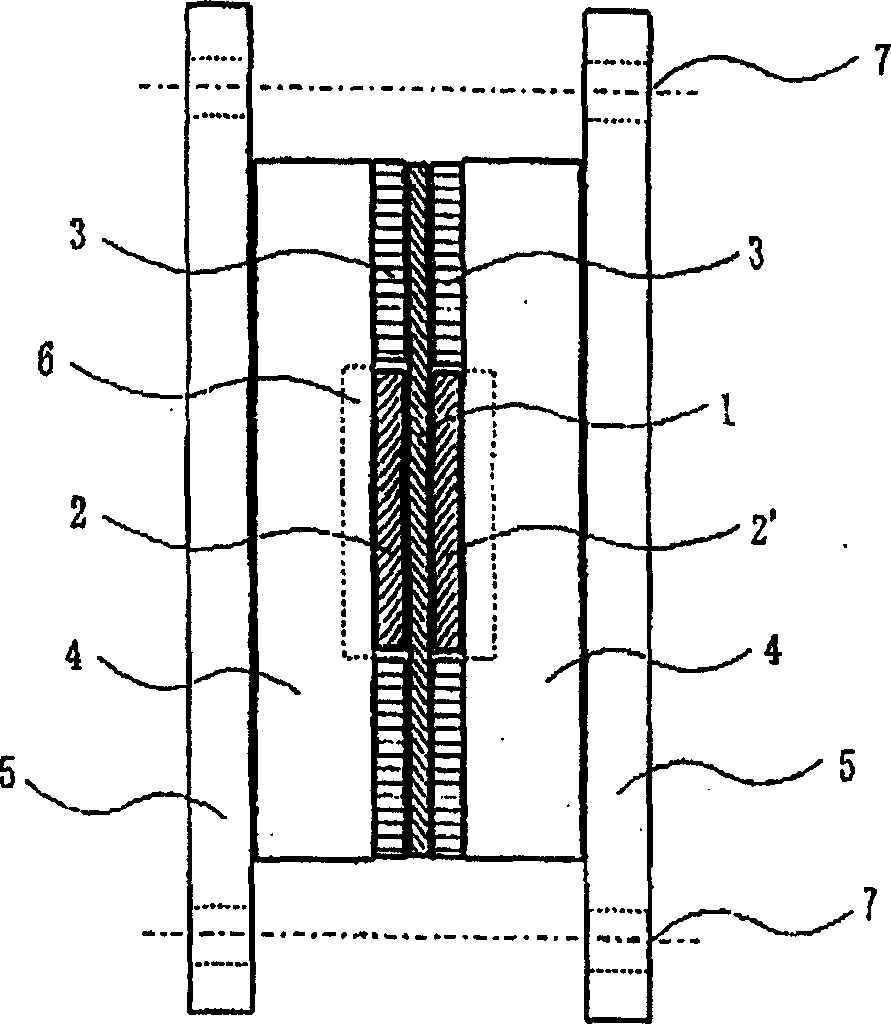
[0127] A proton-acid-group-containing cross-linking aromatic resin having an alkyl group having 1 to 10 carbon atoms directly bonded to a carbonyl group and an aromatic ring is used as a cross-linking group, which is an embodiment of the proton-acid-group-containing cross-linking aromatic resin of the present invention. better choice. Examples of such aromatic resins include aromatic polyethers, aromatic polyamides, aromatic polyimides, aromatic polyamideimides, and aromatic polypyrroles. In particular, aromatic polyethers and aromatic polyamides, which have excellent solvent solubility when not cross-linked and are easy to realize processing such as film formation, are preferred, and are not affected by hot water, acid, alkaline water, alcohols, etc. The hydrolysis of aromatic polyethers is particularly suitable. That is, a proton acid group-containing cross-linkable aromatic polyether having an alkyl group having 1 to 10 carbon atoms directly bonded to a carbonyl group and ...
specific example 3
[0229] The cross-linking aromatic resin containing proton acid group with carbon-carbon double bond or carbon-carbon triple bond as the cross-linking group is a better choice for the cross-linking aromatic resin containing proton acid group in the present invention. Examples of such aromatic resins include aromatic polyethers, aromatic polyamides, aromatic polyimides, aromatic polyamideimides, and aromatic polypyrroles. In particular, aromatic polyethers and aromatic polyamides, which have excellent solvent solubility when not crosslinked and are easy to process such as molding, are preferred, and are not affected by hot water, acid, alkaline water, alcohols, etc. Hydrolyzed aromatic polyethers are particularly suitable. That is, a proton acid group-containing cross-linkable aromatic polyether having a carbon-carbon double bond or a carbon-carbon triple bond is particularly suitable.
[0230] In the present invention, cross-linking aromatic resins containing proton acid group...
Embodiment
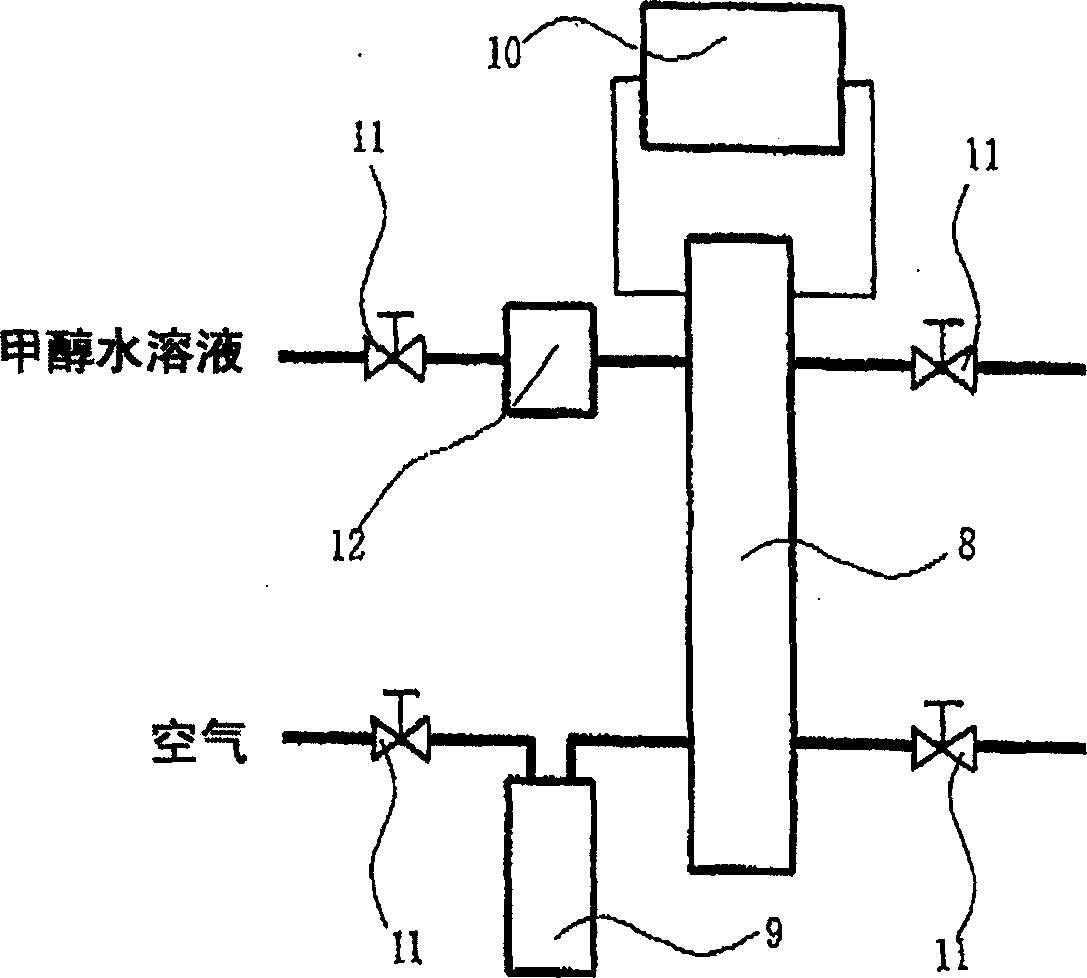
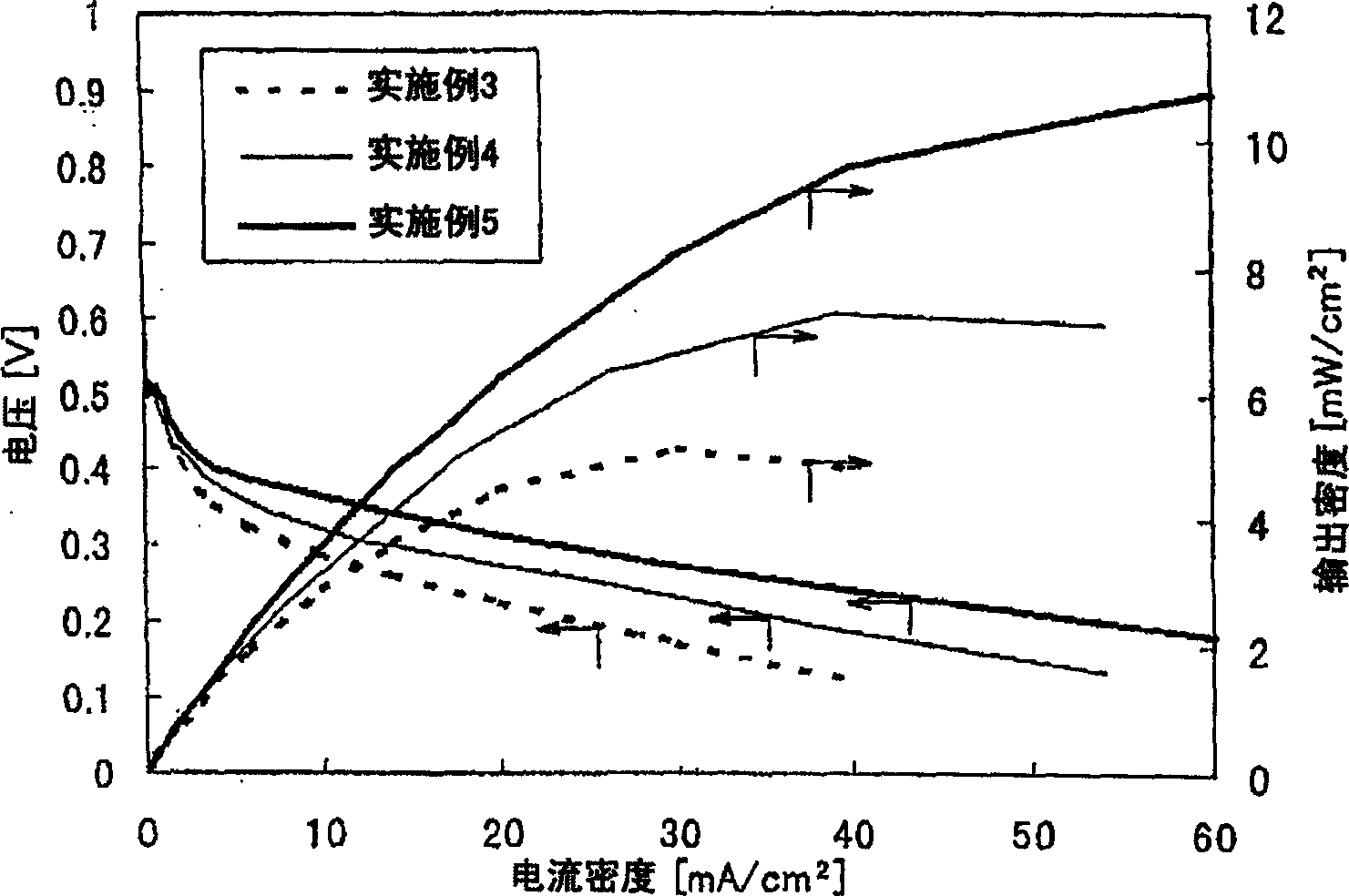
[0394] The present invention will be further described in detail below according to the examples, but the present invention is not limited by the examples. Various test methods in Examples are as follows.
[0395] (i) Reduced viscosity
[0396] Dissolve 0.50 g of polymer powder in 100 ml of solvent, or dilute the polymer solution to 0.005 g / ml, and measure at 35°C.
[0397] (ii) 5% weight loss temperature, weight loss start temperature
[0398] In air, DTA-TG (TG-DTA2000 manufactured by Mac Science Co., Ltd.) was used for measurement at a temperature increase rate of 10° C. / min.
[0399] (iii) Glass transition temperature
[0400] The temperature was measured up to 400° C. at a heating rate of 10° C. / min using a differential scanning calorimeter (DSC3100 manufactured by Mac Syences Co., Ltd.).
[0401] (iv) Proton exchange
[0402] According to the following method, the metals of uric acid can be restored to free protonic acid.
[0403] (1) The powder, film, or photocros...
PUM
| Property | Measurement | Unit |
|---|---|---|
| glass transition temperature | aaaaa | aaaaa |
| transition temperature | aaaaa | aaaaa |
| glass transition temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com