Benzamides and compositions benzamides for use as fungicizide
A composition and compound technology, applied in the direction of fungicides, biocides, biocides, etc., can solve the problems of production decline and consumer cost increase
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0069]
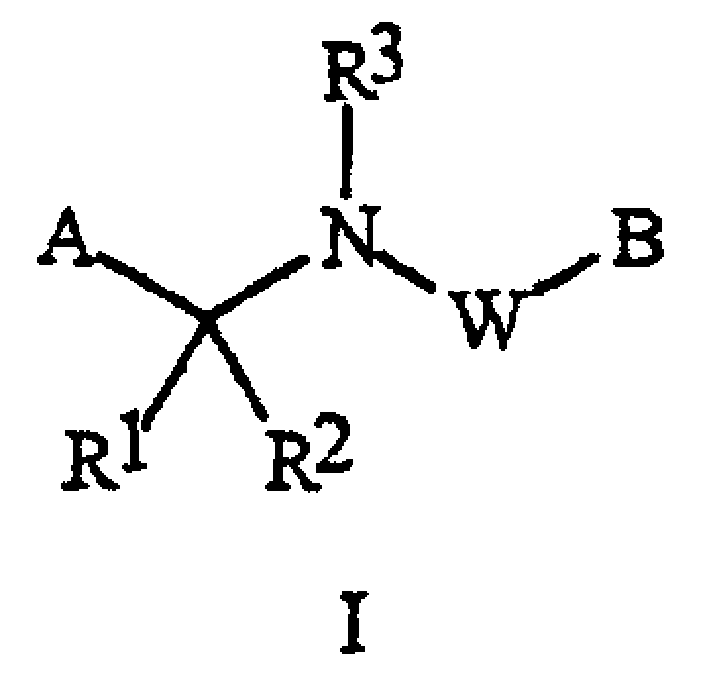
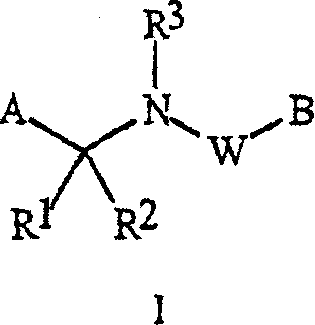
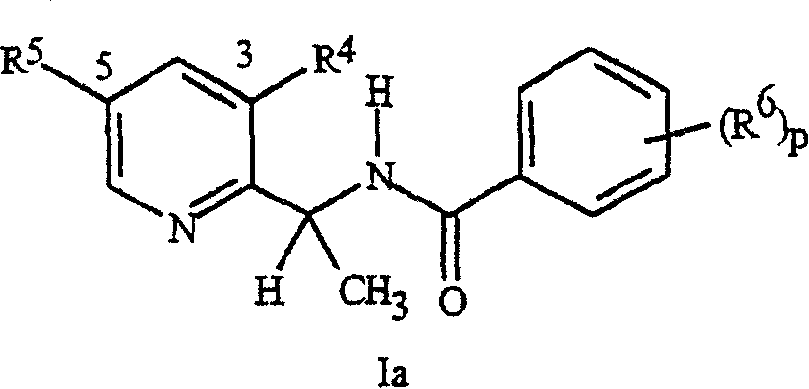
[0070] When R 5 connected to A and R 6 R when connected to B 5 and R 6 Examples of include:
[0071] every R 5 and R 6 independently is C 1 -C 6 Alkyl, C 2 -C 6 Alkenyl, C 2 -C 6 Alkynyl, C 3 -C 6 Cycloalkyl, C 1 -C 6 Haloalkyl, C 2 -C 6 Haloalkenyl, C 2 -C 6 Haloalkynyl, C 3 -C 6 Halocycloalkyl, Halogen, CN, CO 2 H, CONH 2 , NO 2 , hydroxyl, C 1 -C 4 Alkoxy, C 1 -C 4 Haloalkoxy, C 1 -C 4 Alkylthio, C 1 -C 4 Alkylsulfinyl, C 1 -C 4 Alkylsulfonyl, C 1 -C 4 Haloalkylthio, C 1 -C 4 Haloalkylsulfinyl, C 1 -C 4 Haloalkylsulfonyl, C 1 -C 4 Alkylamino, C 2 -C 8 Dialkylamino, C 3 -C 6 Cycloalkylamino, C 2 -C 6 Alkylcarbonyl, C 2 -C 6 Alkoxycarbonyl, C 2 -C 6 Alkylaminocarbonyl, C 3 -C 8 Dialkylaminocarbonyl or C 3 -C 6 Trialkylsilyl; or
[0072] every R 5 and R 6 independently phenyl, benzyl, phenoxy, 5- or 6-membered heteroaryl ring or 5- or 6-membered non-aromatic heterocycle, each ring optionally substituted with ...
example 2
[0078]
[0079] In the above description, the term "alkyl" used alone or in compound words such as "alkylthio" or "haloalkyl" includes straight chain or branched chain alkyl groups such as methyl, ethyl, n-propyl , isopropyl, or the different isomers of butyl, pentyl, or hexyl. "Alkenyl" includes straight chain or branched alkenyl groups such as ethenyl, 1-propenyl, 2-propenyl, and the different isomers of butenyl, pentenyl and hexenyl. "Alkenyl" also includes polyenes such as 1,2-propadienyl and 2,4-hexadienyl. "Alkynyl" includes straight or branched alkynes such as ethynyl, 1-propynyl, 2-propynyl and the different isomers of butynyl, pentynyl and hexynyl. "Alkynyl" may also include alkynes containing multiple triple bonds such as 2,5-hexadiynyl. "Alkoxy" includes, for example, methoxy, ethoxy, n-propoxy, isopropoxy and different isomers of butoxy, pentyloxy and hexyloxy. "Alkoxyalkyl" means an alkyl group substituted with an alkoxy group. Examples of "alkoxyalkyl" inc...
Embodiment A
[0254] wettable powder
[0255] Active ingredient 65.0%
[0256] Laurylphenol Polyethylene Glycol Ether 2.0%
[0257] Sodium lignosulfonate 4.0%
[0258] Sodium aluminosilicate 6.0%
[0259] Montmorillonite (sintered) 23.0%
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com