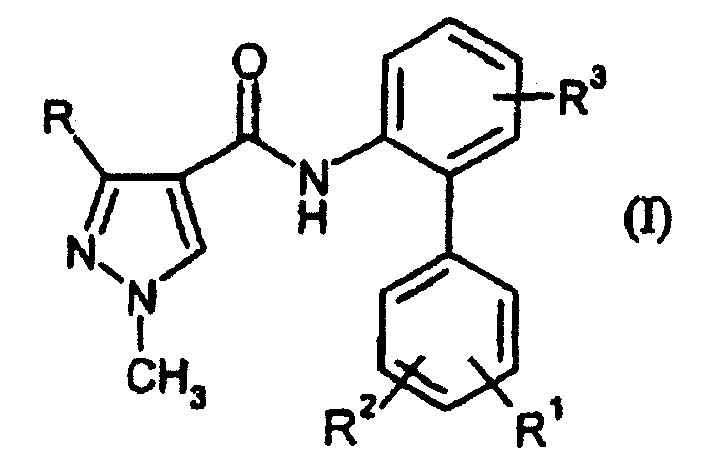
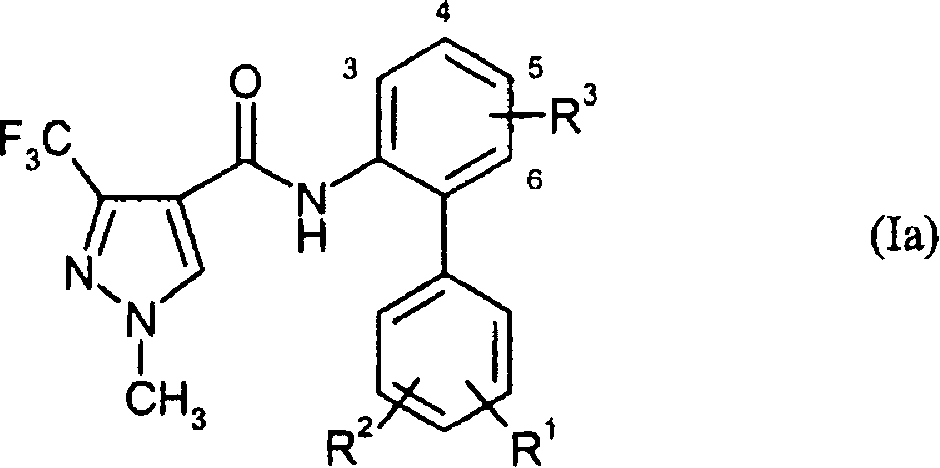
Disubstituted pyrazolyl carboxanilides
A kind of technology of pyrazolyl carboxanilide and compound, which is applied in the application field of preventing and controlling harmful microorganisms, and can solve problems such as unsatisfactory activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0325]
[0326] Method a)
[0327] 0.333g (1.3mmol) 3',4'-dichloro-3-fluoro-1,1'-biphenyl-2-amine and 0.33g (1.56mmol) 1-methyl-3-(trifluoromethyl )-1H-pyrazole-4-carbonyl chloride was dissolved in 6ml of tetrahydrofuran, and 0.36ml (2.6mmol) of triethylamine was added. The reaction solution was stirred at 60°C for 16 hours. For work-up, the mixture was concentrated and the residue was purified by silica gel chromatography, eluting with cyclohexane / ethyl acetate.
[0328] 0.39 g (yield 72% of theory) of N-(3',4'-dichloro-3-fluoro-1,1'-biphenyl-2-yl)-1-methyl-3- (Trifluoromethyl)-1H-pyrazole-4-carboxamide with logP (pH2.3) = 3.10.
Embodiment 2
[0330]
[0331] Method b)
[0332] Oxygen was driven off, and under an argon atmosphere, 0.256 g (0.7 mmol) of N-(2-bromo-6-fluorophenyl)-1-methyl-3-(trifluoromethyl)-1H-pyrazole-4 -Formamide and 0.12 g (0.77 mmol) of 3-chloro-4-fluorophenylboronic acid are suspended in a mixture of 8 ml of toluene, 1.5 ml of ethanol and 5.25 ml of saturated sodium carbonate solution. To the reaction mixture was added a catalytic amount (0.01-0.3 equiv) of tetrakis(triphenylphosphine)palladium(0), and the mixture was heated at 100° C. for 1 hour under an argon atmosphere. The organic phase was separated off and the aqueous phase was extracted with ethyl acetate. The combined organic phases are concentrated and the residue is purified by silica gel chromatography, eluting with cyclohexane / ethyl acetate (1:1).
[0333] 0.27 g (yield 96% of theory) of N-(3'-chloro-3,4'-difluoro-1,1'-biphenyl-2-yl)-1-methyl-3- (Trifluoroethyl)-1H-pyrazole-4-carboxamide, logP(pH2.3)=3.04.
[0334] Following ...
Embodiment (III-1
[0339]
[0340] method d)
[0341] Oxygen was driven off, and under an argon atmosphere, 51.2 g (0.268 mol) of 3,4-dichlorophenylboronic acid and 42.5 g (0.223 mol) of 2-bromo-6-fluoroaniline were suspended in 300 ml of toluene, 30 ml of ethanol and 220 ml of saturated in a mixture of sodium carbonate solution. To the reaction mixture was added 2.6 g of tetrakis(triphenylphosphine)palladium(0), and the mixture was heated at 80° C. for 12 hours. The organic phase was separated off and the aqueous phase was extracted with ethyl acetate. The combined organic phases are concentrated and the residue is purified by silica gel chromatography, eluting with cyclohexane / ethyl acetate (3:1).
[0342] 37.4 g (yield 65% of theory) of 3',4'-dichloro-3-fluoro-1,1'-biphenyl-2-amine with logP (pH 2.3)=4.09 are obtained.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com