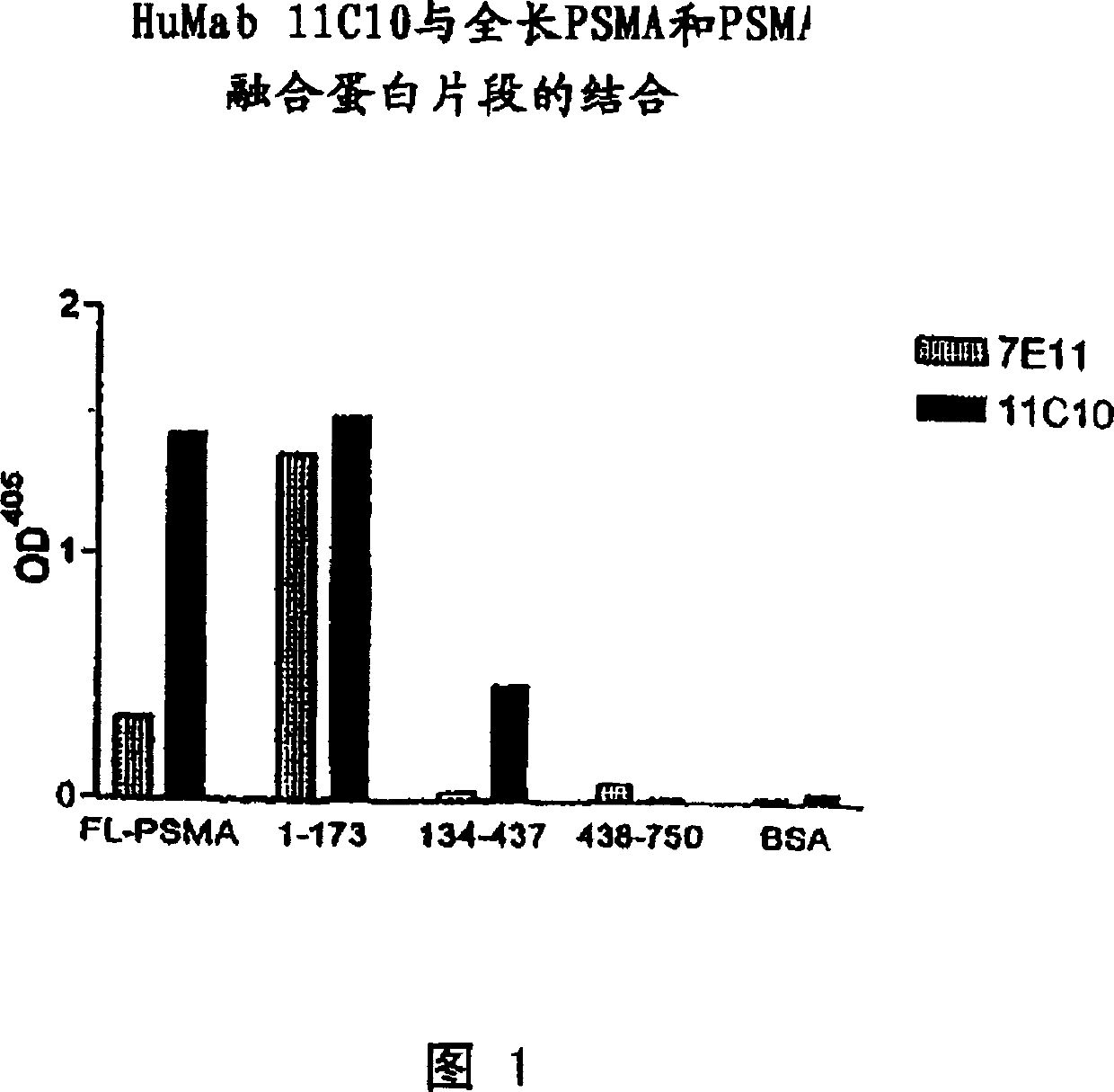
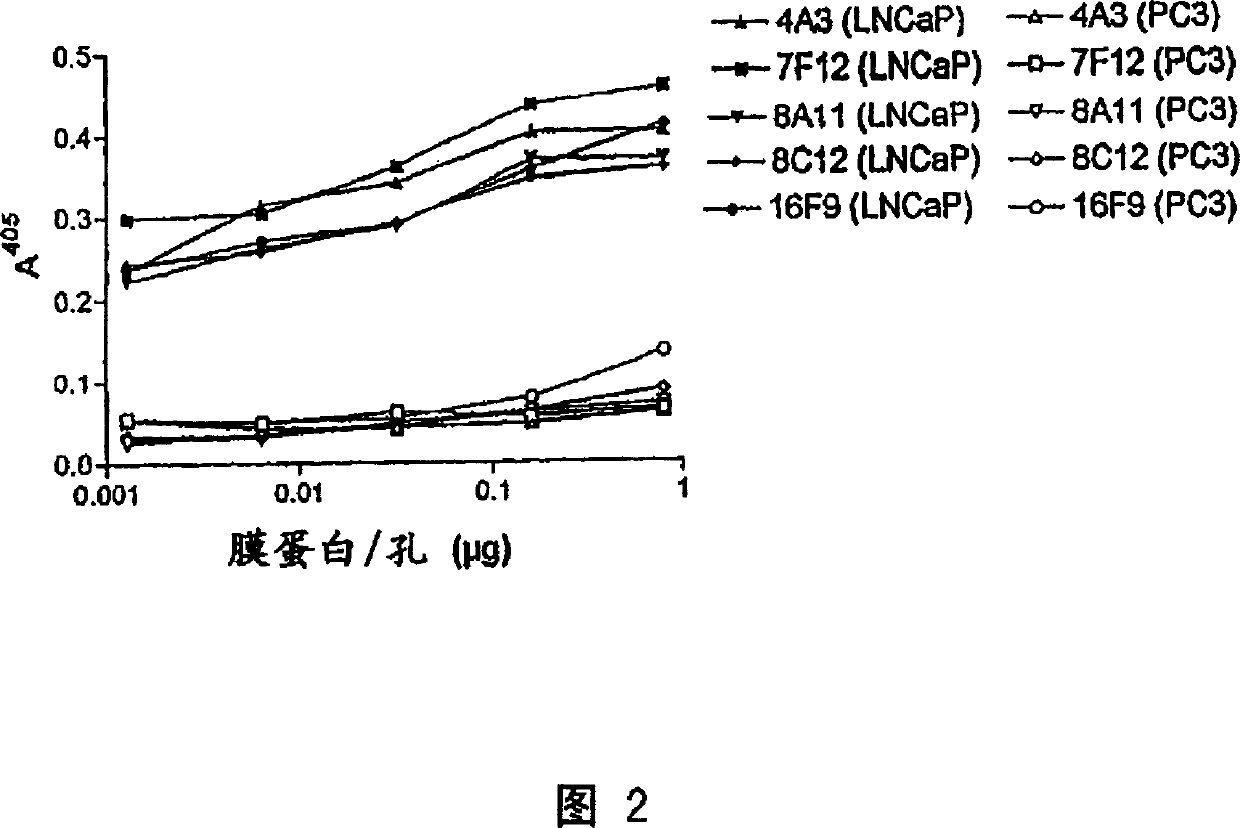
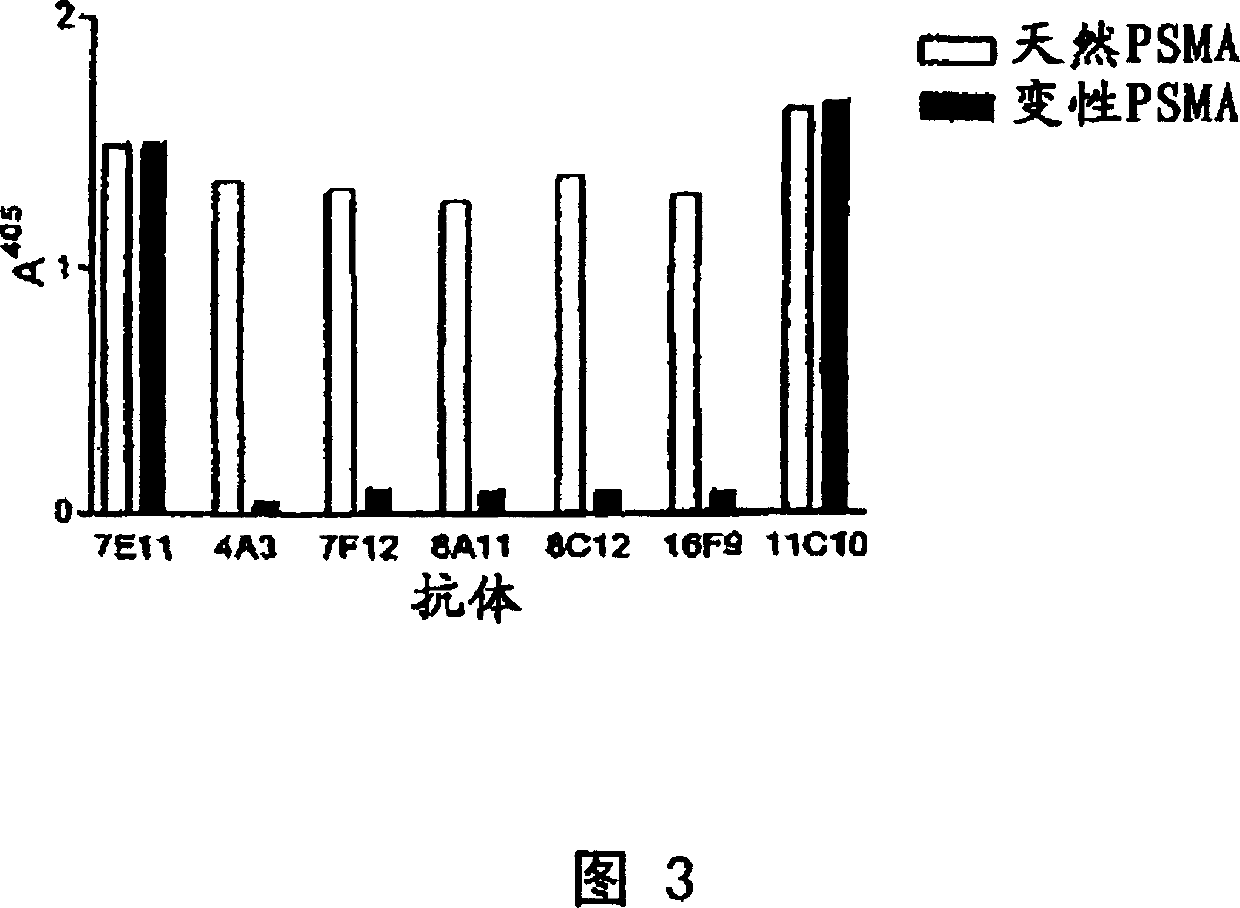
Human monoclonal antibodies to prostate specific membrane antigen (PSMA)
A human monoclonal antibody, human antibody technology, applied in the direction of antibody, anti-receptor/cell surface antigen/cell surface determinant immunoglobulin, anti-animal/human immunoglobulin, etc., can solve the problem of indistinguishable benign prostatic hyperplasia Prostate cancer and other issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0284] Example 1 Generation of Cmu-targeted mice for the production of anti-PSMA human antibodies
[0285] Construction of CMD targeting vector:
[0286] Plasmid pICEmu contains an EcoRI / XhoI fragment spanning the murine Ig heavy chain locus of the mu gene obtained from the Balb / C genomic lambda phage library (Marcu et al. Cell 22:187, 1980). This genomic fragment was subcloned into the XhoI / EcoRI site of plasmid pICEMI9H (Marsh et al.; Gene 32, 481-485, 1984). The heavy chain sequence included in PICEmu is amplified from an EcoRI site located 3′ downstream of the mu intronic enhancer to an XhoI site approximately 1 kb downstream of the last transmembrane exon of the mu gene; Passaging in Bacillus deleted most of the mu-switched repeat regions.
[0287] The targeting vector was constructed as follows. A 1.3 kb HindIII / SmaI fragment was excised from pICEmu and subcloned into HindIII / SmaI digested pBluescript (Stratagene, LaJolla, CA). This pICEmu fragment was amplified fr...
Embodiment 2
[0297] Example 2 Generation of HCO12 Transgenic Mice for Anti-PSMA Human Antibody Production
[0298] HCO12 Human Heavy Chain Transgene:
[0299] The HCO12 transgene was generated by co-injecting the 80 kb insert of pHC2 (Taylor et al., 1994, Int. Immunol., 6:579-591 ) and the 25 kb insert of pVx6. Plasmid pVx6 was constructed as described below.
[0300] The 8.5kb HindIII / SalI DNA fragment, including germline human V H The 1-18 (DP-14) gene and approximately 2.5 kb of 5' flanking, and 5 kb of 3' flanking genomic sequence, were subcloned into plasmid vector pSP72 (Promega, Madison, WI), resulting in plasmid p343.7.16.7 kb BamHI / HindlII DNA fragments, including germline human V H The 5-51 (DP-73) gene and approximately 5 kb of 5' flanking, and 1 kb of 3' flanking genomic sequence, were subcloned into a pBR322-based plasmid cloning vector (Taylor et al. 1992, Nucleic Acids Res. 20:6287-6295 ), the plasmid p251f was generated. A new cloning vector derived from pGP1f, pGP1k...
Embodiment 3
[0302] Example 3 Generation of Anti-PSMA Human Monoclonal Antibodies and Bispecificity
[0303] antigen : antigen (Northwest Biotherapeutics, Inc): (1) cell membrane and (2) purified protein (PSMA) isolated from LNCaP cells (Cat # CRL-1740; American Type Culture Collection, Rockville, MD) provided in two forms: ). Purified antigen (1.05 mg / ml) was used for one immunization and a final tail vein boost. Monoclonal antibody 7E11. C5 was obtained from Cytogen, Inc, Princeton, NJ.
[0304] Solubilized PSMA and membranes from LNCaP cells were mixed with complete or incomplete Freund's adjuvant (CFA and IFA). Mice were injected intraperitoneally with 0.2 cc of the prepared antigen. A final tail vein immunization was performed with soluble PSMA in sterile PBS.
[0305] transgenic mice : Mice were housed in mesh cages and assessed for good physiological status on the day of immunization, bleeding and fusion. (CMD)++; (HCo12)15087+; (JKD)++; (KCo5)9272+ genotype Male mouse ID#...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com