Application of erigeron breviscapus aglucon in preparating medicine
A technology of scutellarin and scutellarin, which is applied to the application field of scutellarin aglycone in the pharmaceutical field, can solve the problems of no relevant reports and little research
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
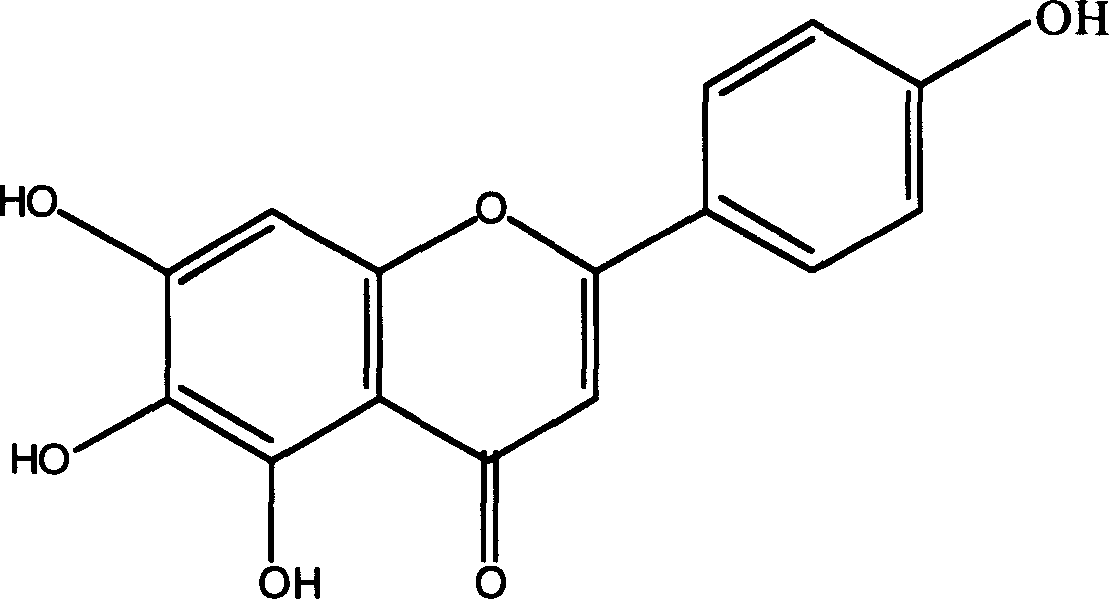
[0051] Use 2.5kg of the whole plant of Erigeron breviscapus Yunnan, add 95% ethanol to extract, dissolve the extract in hot water at 70-80°C, extract with chloroform, then extract with ether and ethyl acetate respectively, recover the solvent, and obtain a yellow precipitate. The precipitate was recrystallized from a mixed solvent of pyridine and water, and then subjected to polyamide column chromatography to obtain 2000 mg of scutellarin aglycon as a light yellow powder.
[0052] Mass spectrometry EI-MS: (M + ) 286; C NMR spectrum 13CNMR: 163.59 (C-2), 103.9 (C-3), 182.0 (C-4), 149.6 (C-5), 129.1 (C-6), 161.0 (C-7) , 93.8(C-8), 147.0(C-9), 102.4(C-10), 128.4(C-1'), 121.5(C-2'), 115.9(C-3'), 153.3(C- 4'), 115.9(C-5'), 121.5(C-6').
[0053] Proton nuclear magnetic resonance spectrum 1H-NMR: 6.73 (s, H-3), 6.57 (s, H-8), 12.77 (s, OH-5), 8.77 (s, OH-6), 10.53 (s, OH- 7), 10.37 (s, OH-4'), 7.89 (d, 2H, H-2', -6'), 6.90 (d, 2H, H-3', 5').
[0054] Breviscapine aglycon oral sol...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com