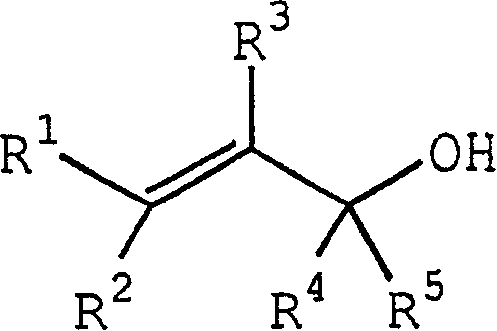
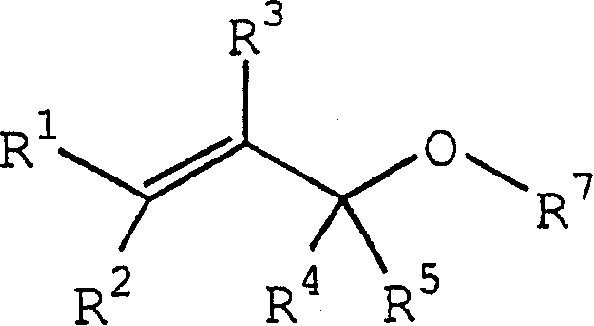
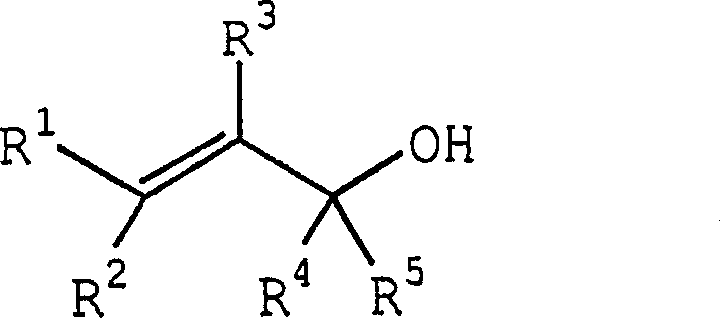
Process for producing ether compounds in presence of a copper (II) salt
A compound and a technology for preparing ethers, which are applied in organic chemistry methods, chemical instruments and methods, and the preparation of ethers by exchanging organic parts, and can solve problems such as preparation methods that have not been reported yet
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0248] Embodiment 1: the etherification reaction of allyl alcohol
[0249] Etherification of allyl alcohol was carried out by using a glass autoclave reaction apparatus (HYPERGLASSTERTEM-V1000N, manufactured by Taiatsu Techno Co., Ltd.) having a volume of 1 L equipped with a stirring shaft coated with Teflon (registered trademark) and a thermocouple for measuring the internal temperature reaction. Into this reactor were charged 350 g (6.03 mol) of allyl alcohol and 10.28 g (60.3 mmol) of copper(II) chloride dihydrate (manufactured by Wako Pure Chemical Industries Co., Ltd., guarantee reagent). The reactor was sealed and an air tightness test was performed using nitrogen to verify that there were no leaks in the system. Thereafter, the pressure in the system was returned to atmospheric pressure, stirring was performed at a rotation speed of 350 rpm, and the power of the heater outside the reactor was turned on to start heating. The time point when the temperature inside the...
Embodiment 2
[0253] Embodiment 2: the etherification reaction of allyl alcohol
[0254] This process was repeated in the same manner as in Example 1, except that the etherification reaction was performed at a reaction temperature of 155° C., and the reaction time was changed to 2.0 hours. The reaction results are shown in Table 1 above.
Embodiment 3
[0255] Embodiment 3: the etherification reaction of allyl alcohol
[0256] The process was carried out in the same manner as in Example 1, except that the amount of copper(II) chloride dihydrate used was 30.84 g (180.9 mmol), and the reaction time was changed to 1.0 hour. The reaction results are shown in Table 1 above.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com