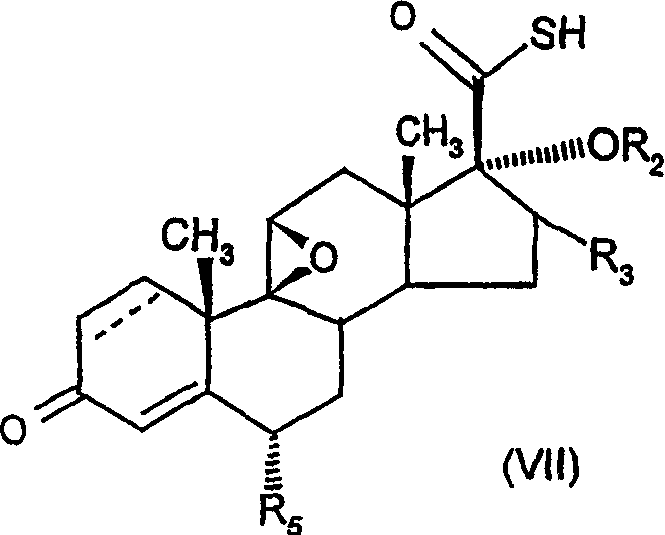
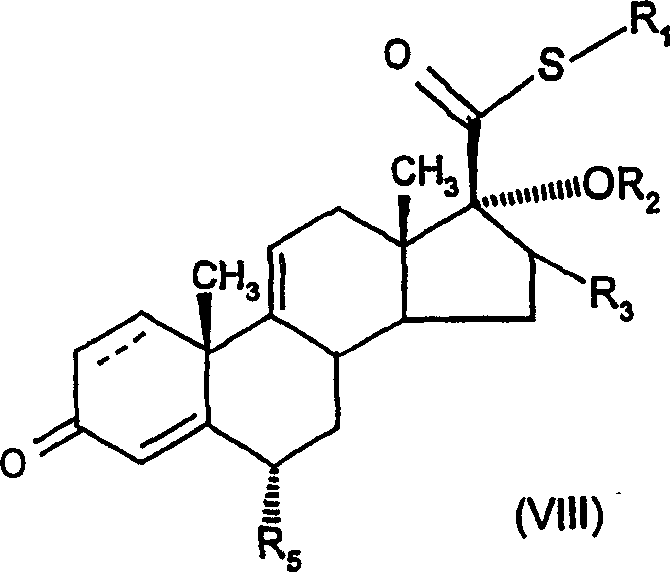
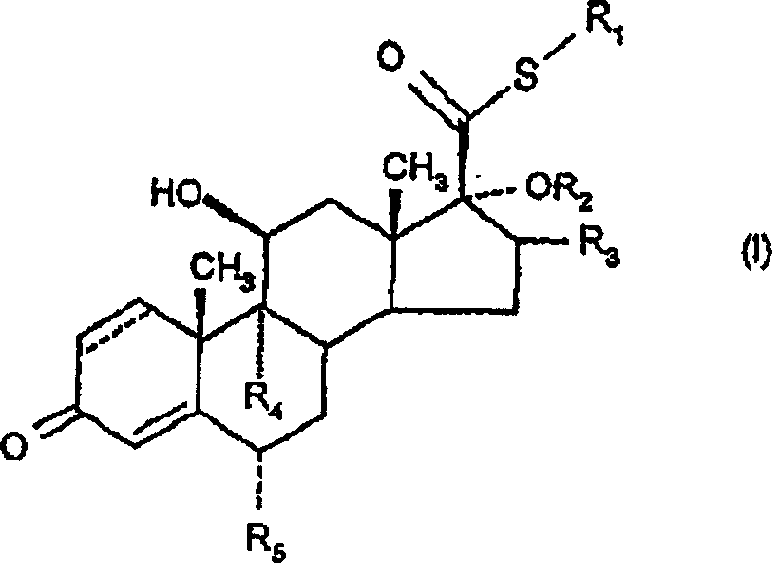
6.alpha., 9.alpha.-difluoro-17.alpha.-'(2-furanylcaboxyl)oxy!-11.beta.-hydroxy-16.alpha.-methyl-3-oxo-androst-1,4,-diene-17-carbothioic acid s-fluoromethyl ester as an anti-inflamatory agent
A technology of substituents and heteroaryls, applied in the field of androstane series compounds, can solve the problems of difficult understanding of pharmacodynamics and pharmacokinetics
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0299] Example 1: 6α,9α-difluoro-17α-[(2-furylcarbonyl)oxy]-11β-hydroxy-16α-methyl- S-fluoromethyl 3-oxo-androst-1,4-diene-17β-thiocarboxylate
[0300] Will intermediate A suspension of 1 (2.5g, 4.94mmol) was dissolved in dry N,N-dimethylformamide (25ml) and sodium bicarbonate (465mg, 5.53mmol) was added. The mixture was stirred at -20°C and bromofluoromethane (0.77ml, 6.37mmol) was added and the mixture was stirred at -20°C for 2 hours. Diethylamine (2.57ml, 24.7mmol) was added, and the mixture was stirred at -20°C for 30 minutes. The mixture was added to 2M hydrochloric acid (93ml) and stirred for 30 minutes. Water (300ml) was added and the precipitate was collected by filtration, washed with water and dried under vacuum at 50°C to give a white solid which was recrystallized in acetone / water and dried under vacuum at 50°C to give Title compound (2.351g, 88%):
[0301] LCMS retention time 3.66 min, m / z 539 MH + , NMRδ (CDCl 3 ) including 7.60 (1H, m), 7.18-7.11 (...
Embodiment 2
[0302] Example 2: 6α,9α-difluoro-17α-[(3-furylcarbonyl)oxy]-11β-hydroxy-16α-methyl- S-fluoromethyl 3-oxo-androst-1,4-diene-17β-thiocarboxylate
[0303] Use something like Example 1 The method described by Intermediate 2 to prepare Example 2 .
[0304] LCMS retention time 3.72 minutes, m / z 539 MH + .
Embodiment 3
[0305] Example 3: 6α,9α-difluoro-11β-hydroxy-16α-methyl-3-oxo-17α-[(2-thienylcarbonyl) Oxy]-androst-1,4-diene-17β-thiocarboxylate S-fluoromethyl ester
[0306] Use something like Example 1 The method described by Intermediate 3 to prepare Example 3 .
[0307] LCMS retention time 3.81 min, m / z 555 MH + .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com