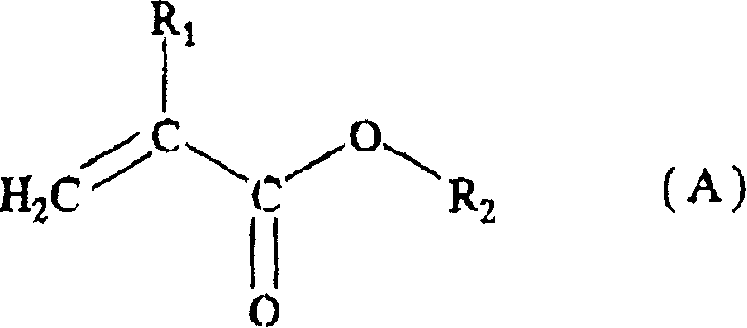
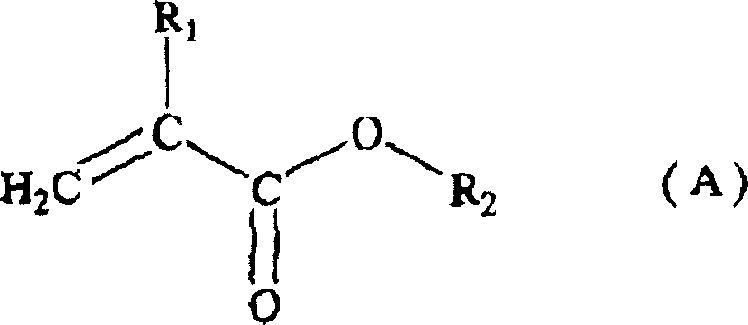
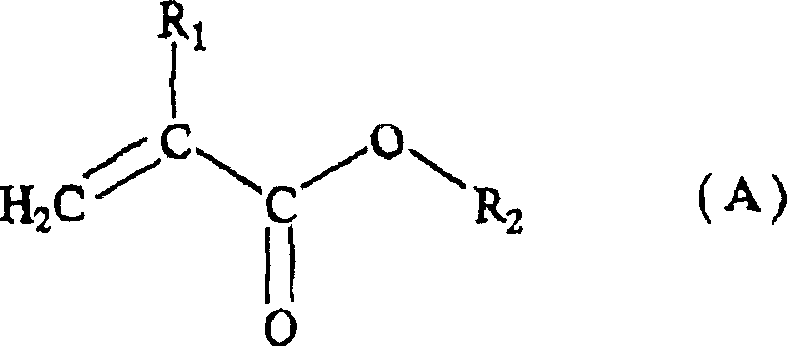
Acrylic resin
An acrylic resin and alicyclic technology, applied in the direction of synthetic resin layered products, adhesive types, film/sheet adhesives, etc., can solve problems such as light leakage, curling and peeling of optical lamination films
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0154]
[0155] The acrylic resin solution prepared in Polymerization Example 1 was used as the solution of the acrylic resin (1). 150 parts of ethyl acetate were added to 100 parts of non-volatile components in the acrylic resin (1) to prepare an ethyl acetate solution of an acrylic resin composition having a non-volatile component content of 19.5%. In 100 parts of nonvolatile components in the resulting solution, 0.8 parts of a polyisocyanate-based compound (trade name: Takenate D-160N, manufactured by Mitsui-Takeda Chemical Inc.) and 0.4 parts of a silane-based compound (trade name Name: KBM-403, manufactured by Shin-Etsu Silicone) was used as a crosslinking agent to prepare the adhesive of the present invention.
[0156]
[0157]Using an applicator, the adhesive thus prepared was coated on the surface of a release-treated polyethylene terephthalate film (manufactured by LINTEC Corporation, trade name: PET3811), which had been subjected to a release treatment. , so the...
Embodiment 2 to 8 and comparative Embodiment 1 to 3
[0178] According to Example 1, using the acrylic resins (1) and (2) in the weights shown in Table 2, acrylic resin compositions, adhesives, optical laminate films and optical laminates were prepared. The obtained optical laminate was evaluated in the same manner as in Example 1, and the results are shown in Table 2 together with the results of Example 1.
[0179] In Comparative Examples 1 and 2, an adhesive composed only of the acrylic resin (2), that is, an adhesive composed of an acrylic resin not containing the structural unit (b) was used, and in Comparative Example 3, An adhesive composed of an acrylic resin (2) composition was used.
[0180] Example
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com