Curing and preventing method
A technology of reagents and receptors, applied in the field of chronic immune-mediated inflammatory diseases, dynamics and/or screening of animal models of the reagents, can solve problems such as glomerulonephritis that cannot be prevented
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
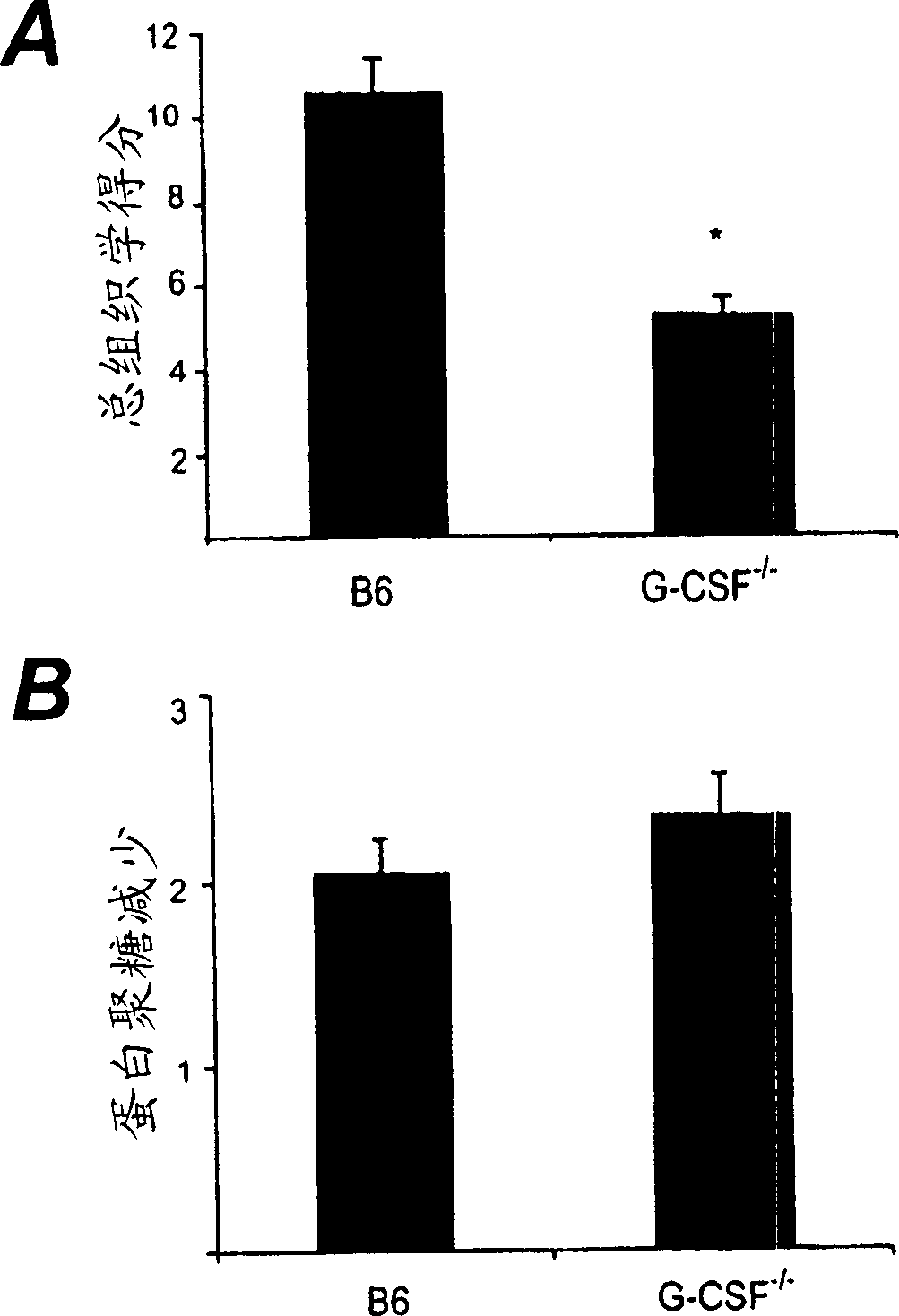
[0112] Mouse
[0113] C57BL / 6 (B6; wild type, [WT]) mice were obtained from Walter and Eliza Hall Institute (WEHI) Animal Supplies (Victoria, Australia). G-CSF-defective (G-CSF - / -) Mice were obtained from Ludwig Institute for Cancer Research, Victoria, Australia, and were produced by directed destruction of the Cysf3 gene on 129 / OLA embryonic stem (ES) cells, and injected the gene into B6 blastocysts (Lieschke et al. , Blood 84: 1737-1746, 1994). The mice were backcrossed to the B6 background for more than 20 generations. All mice are: ≥8 weeks of age at the time of the experiment, fed standard rodent feed, and drank water ad libitum, and were housed (≤6 mice / cage) in a cage containing sawdust. All animal methods were approved by the Institutional Ethics Committee.
Embodiment 2
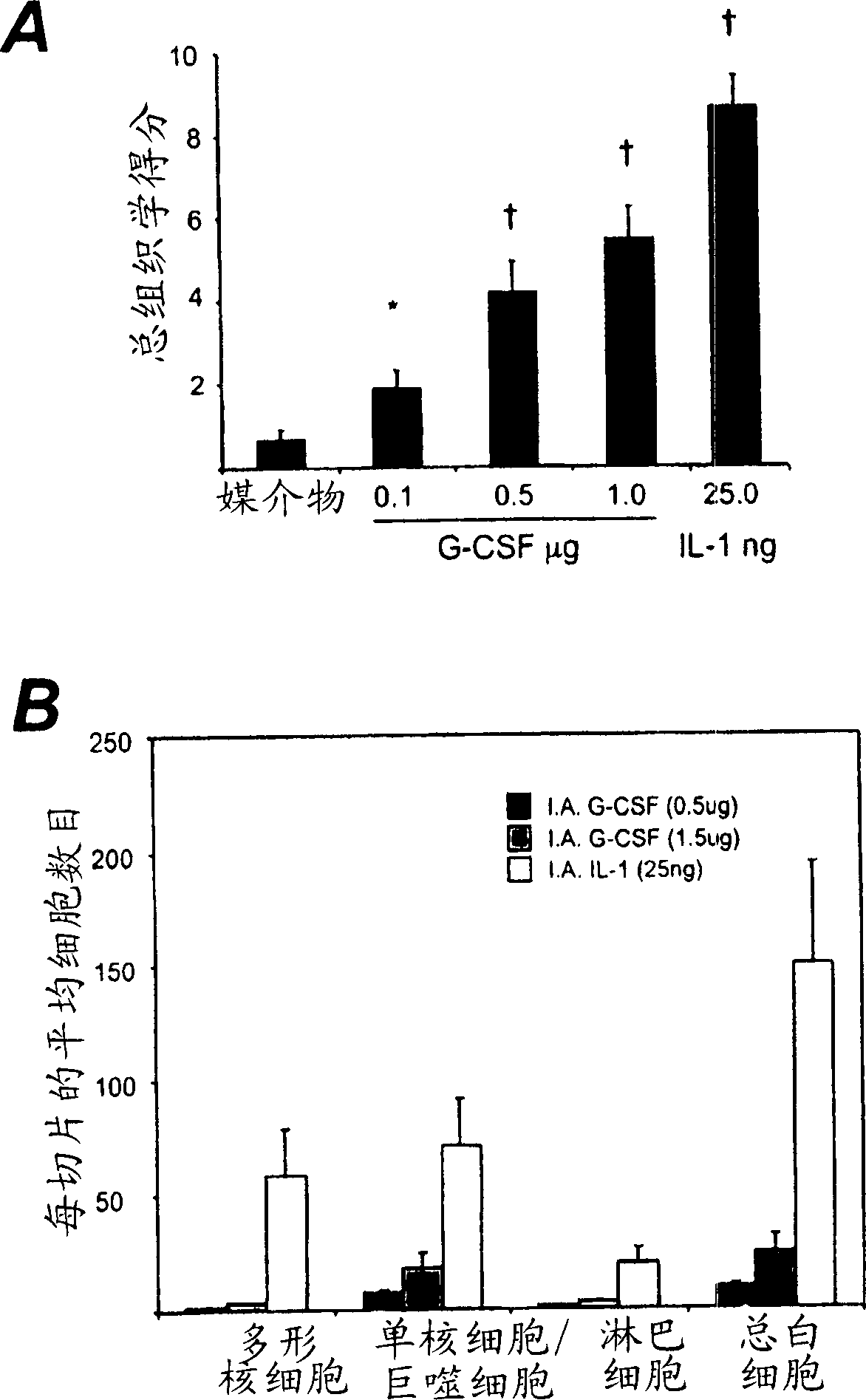
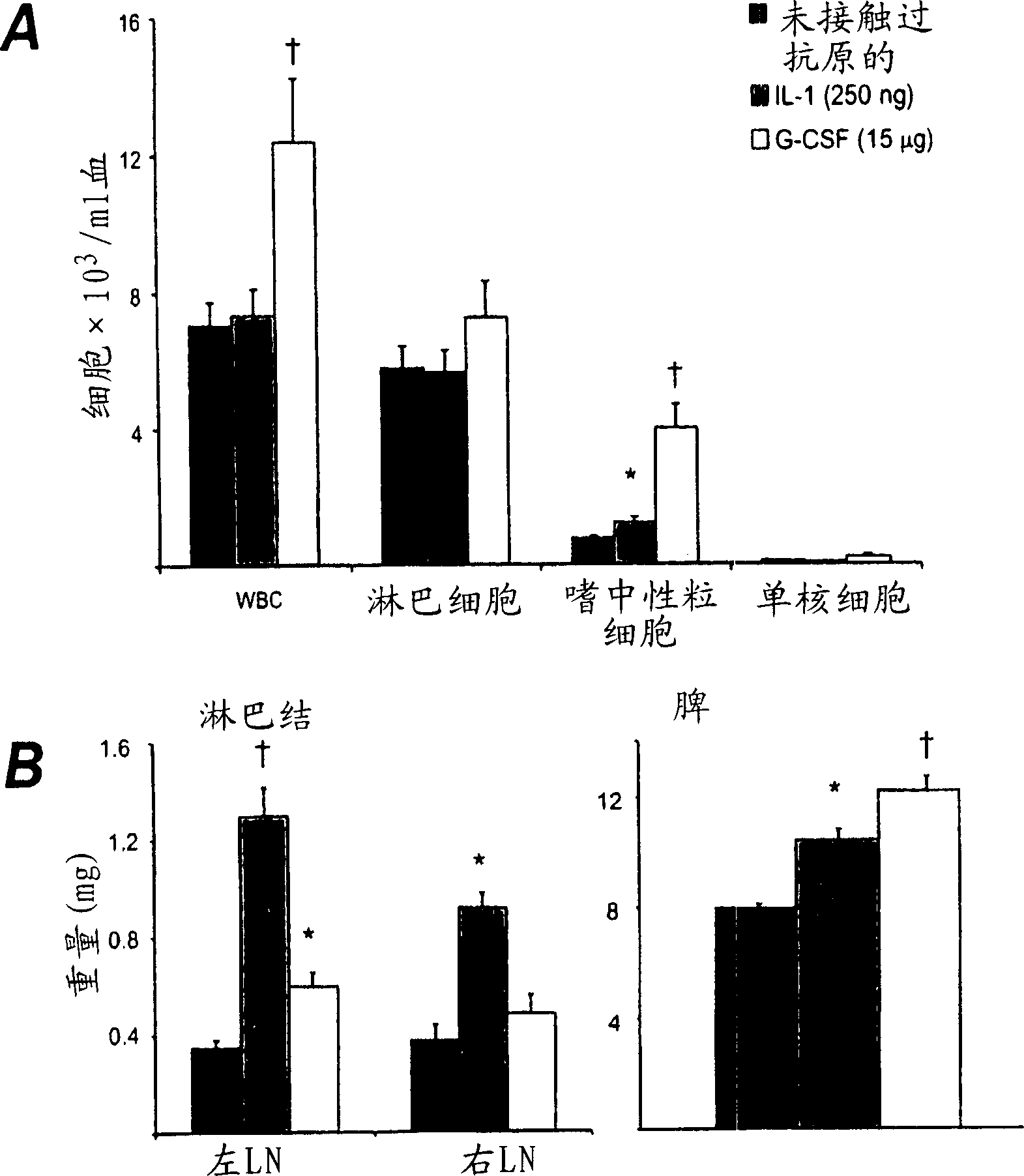
[0115] Induction of mBSA / IL-1-induced arthritis (acute arthritis)
[0116] This method is based on a previously described technique (Lawlor et al., Arthritis and Rheumatism 44:442-450, 2001). The mice were anesthetized, and 10 μl of 20 mg / ml mBSA (Sigma, St Louis, MO) was injected into the knee joint by the intra-articular route. The control joints received the same volume of vehicle (normal saline). Then 20μl of 12.5μg / ml recombinant human IL-1β (specific activity 5×10) in normal saline / 0.5% (v / v) normal mouse serum (vehicle) was injected subcutaneously (s.c.) 8 U / mg; Amgen, Thousand Oaks, CA) was injected into the hind paw pad of the mouse, and the injection was repeated on the second day.
[0117] On the 7th day (or at the indicated time point) the mice were slaughtered, and the knee joints removed, and fixed in 10% (v / v) neutral buffered formalin solution for at least 2 days, decalcified and performed Paraffin treatment. Frontal tissue sections (4 μm) were cut at four dep...
Embodiment 3
[0120] Induction of collagen-induced arthritis (CIA)
[0121] Chicken type II collagen (CII; Sigma) was dissolved in 10 mM acetic acid at a concentration of 2 mg / ml overnight at 4°C, and emulsified in an equal volume of Freund’s complete adjuvant (CFA). The agent was prepared to a concentration of 5 mg / ml by adding heat-inactivated Mycobacterium taberculosis (strain H37 Ra; Difco Laboratories, Detroit, MI, USA) to Freund's incomplete adjuvant (Difco). 100 μl of the emulsion was injected into several parts of the tail base of the mouse by intradermal (i.d.) injection, and the above injection was repeated 21 days later.
[0122] The animals were monitored for erythema and limb swelling, and each mouse was clinically scored 3 times a week until 40 days. The scoring system is as described above (Campbell et al., European Journal of Immunology 30:1568-1575), where 0=normal, 1=slight swelling, 2=severe swelling, and 3=joint deformity and / or stiffness, and each mouse The maximum ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com