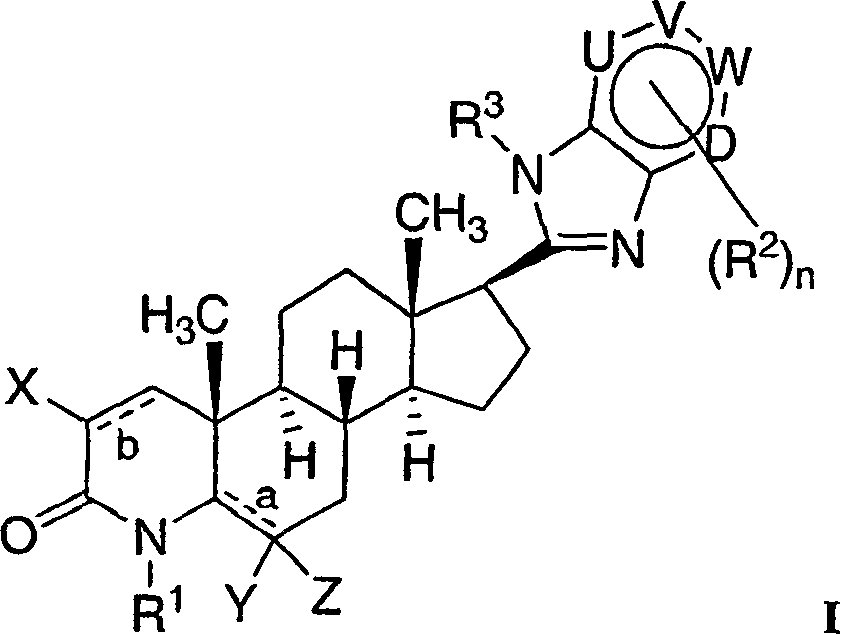
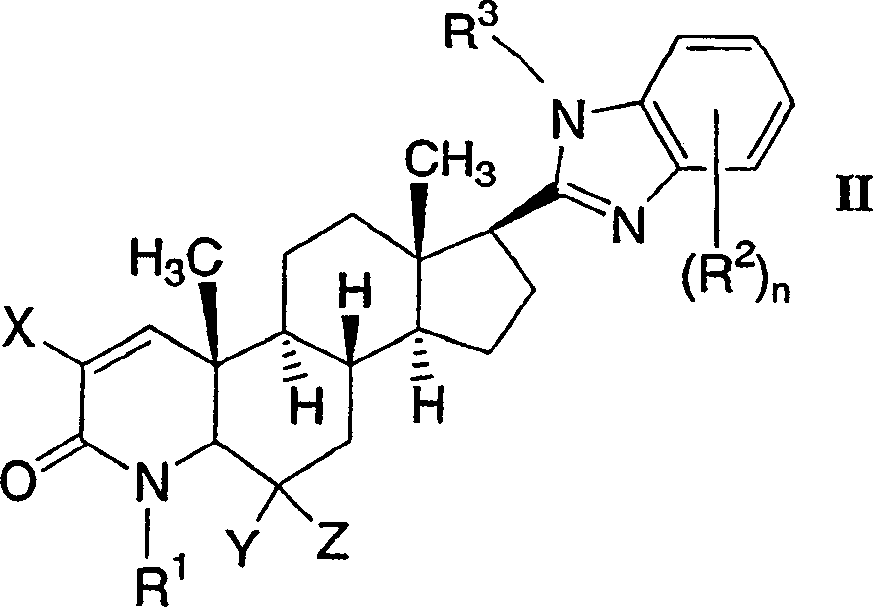
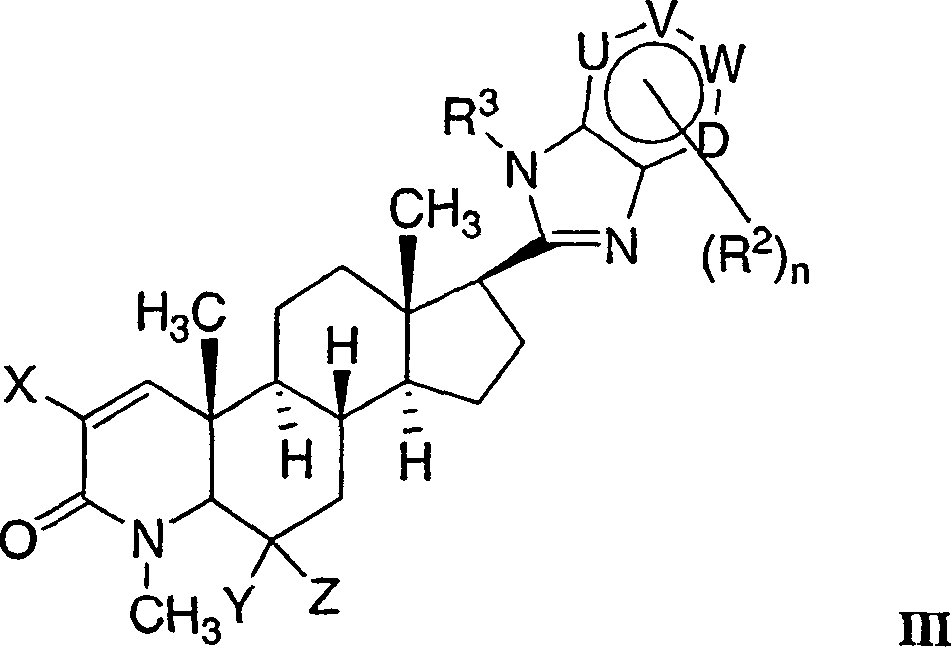
17-heterocyclic-4-azasteroid derivatives as androgen receptor modulators
A heterocyclyl, halogen technology, applied in the field of tissue-selective androgen receptor modulators, can solve problems such as reduced ability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0761] Step A: N-(3-Amino-pyridin-2-yl)-4-methyl-3-oxo-4-aza-5α-androst-1-ene-17β-carboxamide (1-4)
[0762] at room temperature 1-1 (10.0 g, 23.6 mmol) in dichloroethane were added silver triflate (18.2 g, 70.7 mmol) and 2,3-diaminopyridine (7.7 g, 70.7 mmol). The reaction was heated to reflux and stirred for 12 hours. The reaction was cooled, diluted with MeOH, filtered and concentrated. The residue was purified by flash chromatography (0-20% MeOH in EtOAc) to give 1-4 , which is a gray solid.
[0763] MS calculated M+H: 423, found 423.
[0764] Step B: 17β-(1H-imidazo[4,5-b]pyridin-2-yl)-4-methyl-4-aza-5α-androst-1-en-3-one (1-5 )
[0765] Will 1-4 (5.0 g, 11.8 mmol) in polyphosphoric acid (30 mL) was heated to 140 °C and stirred for 14 hours. After cooling, the reaction was washed with 1:1 MeOH:CHCl 3 (500mL) diluted with cold water and NaHCO 3 Quenched by washing with saturated solution. The mixture was then washed with CH 2 Cl 2 extracted, washed with brine...
Embodiment 13
[0777] Step A: 17β-(1-methyl-imidazo[4,5-b]pyridin-2-yl)-4-methyl-4-aza-5α-androst-1-en-3-one ( 2-2)
[0778] at room temperature 1-5 (0.10 g, 0.24 mmol) in DMF was added NaH (0.019 g, 0.48 mmol) and iodomethane (0.033 mL, 0.48 mmol). The reaction was stirred at room temperature for 2 hours. The reaction uses saturated NH 4 Aqueous Cl solution was quenched with CH 2 Cl 2 Dilute and wash with brine. The organic layer was dried, filtered and concentrated. The residue was purified by flash chromatography (0-20% MeOH in EtOAc) to give 2-2 , which is a white solid.
[0779] M+H calculated by MS: 419, found 419.
[0780] Examples 14-16 in Table 2 were prepared in a manner analogous to Example 13, but utilizing an appropriate acylating or alkylating reagent.
[0781] Table 2
[0782]
[0783]
[0784] structural formula 3-6 Selective androgen receptor modulators (SARMs) were prepared according to the method shown in Scheme 3. The starting material is 17β-carboxy es...
Embodiment 17
[0793] Step A: 2α-fluoro-4-methyl-3-oxo-4-aza-5α-androstane-17β-carboxylate methyl ester (3-2)
[0794] 20 minutes introverted 3-1 (7.5 g, 21.6 mmol) in THF (100 mL) was added dropwise a 1.5M solution of LDA in THF (17.3 mL, 25.9 mmol) at -78°C and then stirred for 1 hour. Add FN(SO 2 Ph) 2 (10.2 g, 32.4 mmol) in THF (40 mL). After 30 minutes, the cooling bath was removed and the reaction was stirred for 14 hours. Join Et 2 O, the mixture was washed with water, saturated aqueous sodium bicarbonate, brine, and dried (MgSO 4 ) and then concentrated. Chromatography on silica gel (hexane-EtOAc as eluent) gave 3-2 , which is a colorless solid.
[0795] MS calculated M+H: 366, found 366.1.
[0796]Step B: 2-Fluoro-4-methyl-3-oxo-4-aza-5α-androst-1-ene-17β-carboxylate methyl ester (3-3)
[0797] 30 minutes introverted 3-2 (30 g, 82.1 mmol) in THF (400 mL) was added dropwise a 1.5M solution of LDA in THF (71.1 mL, 107 mmol) at -78°C and then stirred for 1 hour. After this ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com