il-17a conjugates and uses thereof
A technology of IL-17A and conjugates, applied in the application, drug combination, fusion polypeptide and other directions, can solve the problems of pathological development and deterioration of interleukin-17A, and achieve the effect of high affinity and long half-life
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0065] Example 1 Mouse monoclonal antibody against human IL-17A
[0066] Monoclonal antibodies to human IL-17A (hIL-17A) were obtained as follows. 6-8 week-old female BALB / c mice (Shanghai Xipuer-Bikay Experimental Animal Co., Ltd., animal production license number: SCXK (Shanghai) 2008-0016) and 6-8 week-old female SJL mice (Beijing Weitong Lihua Experimental Animal Technology Co., Ltd., animal production license number: SCXK (Beijing) 2012-0001) were divided into two groups of high and low doses, with 10 BALB / c mice and 10 SJL mice in each group.
[0067] The high and low dose groups were respectively immunized with a series of natural hIL-17A variants (His-hIL-17A, where hIL The amino acid sequence of -17A can be found in Genbank human IL-17A protein accession number NP-002181, and the expressed protein was purified sequentially with Ni affinity column (Superdex) 75SEC). The inoculation time was the 0th, 14th, 35th, and 56th day.
[0068] On day 0, the high-dose group re...
Embodiment 2
[0085] Example 2 Humanization of murine anti-human IL-17A antibody
[0086] The humanization of the murine anti-human IL-17A monoclonal antibody mAb049 was basically carried out according to the methods published in many documents in the field. Briefly, humanization is performed by replacing the parental (murine antibody) constant domains with human constant domains, and selecting human antibody sequences based on the homology between the murine and human antibodies.
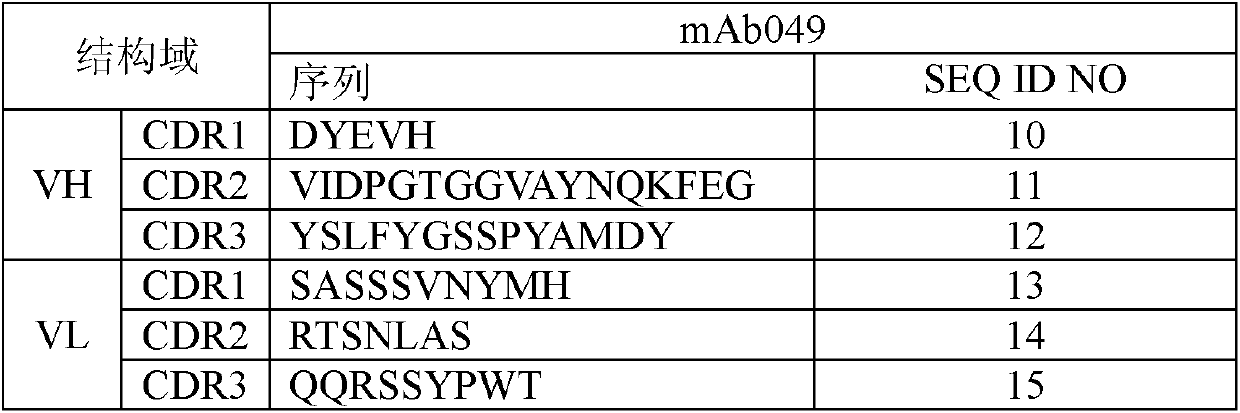
[0087] 1. CDR region of murine anti-IL-17A antibody
[0088] The amino acid residues of VH / VL CDRs are determined and annotated by the Kabat numbering system. The CDR sequence of murine mAb049 in the present invention is described in the following table:
[0089] Table 1: CDR sequences of murine anti-IL-17A antibodies
[0090]
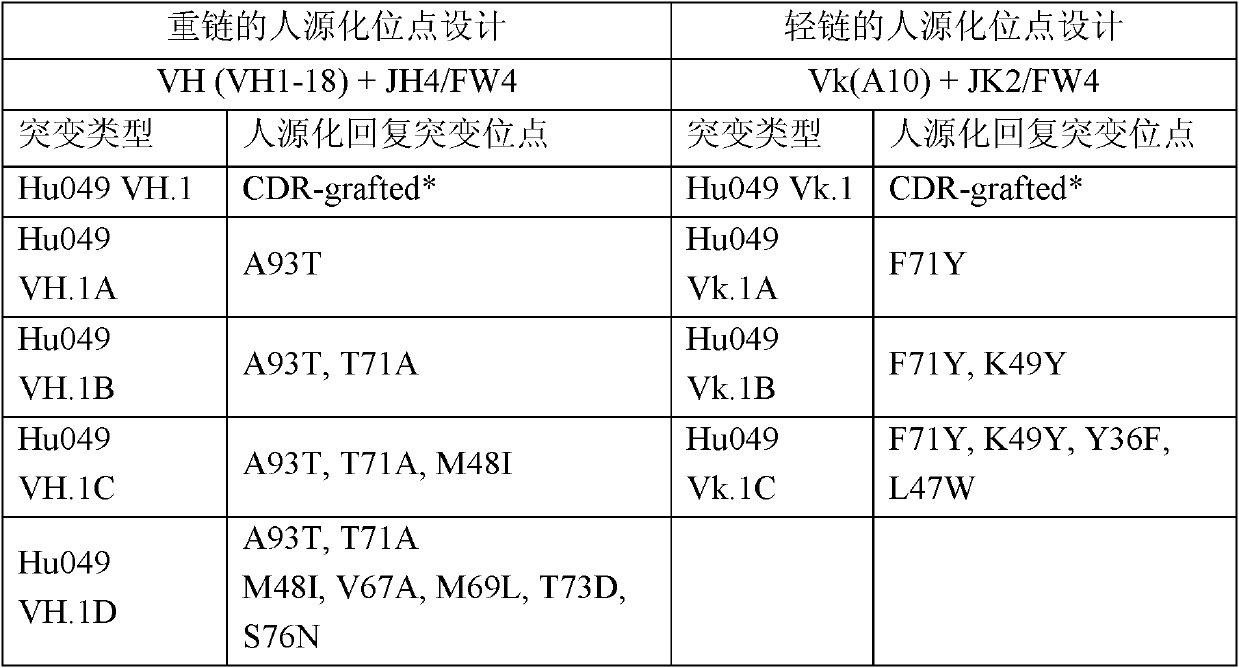
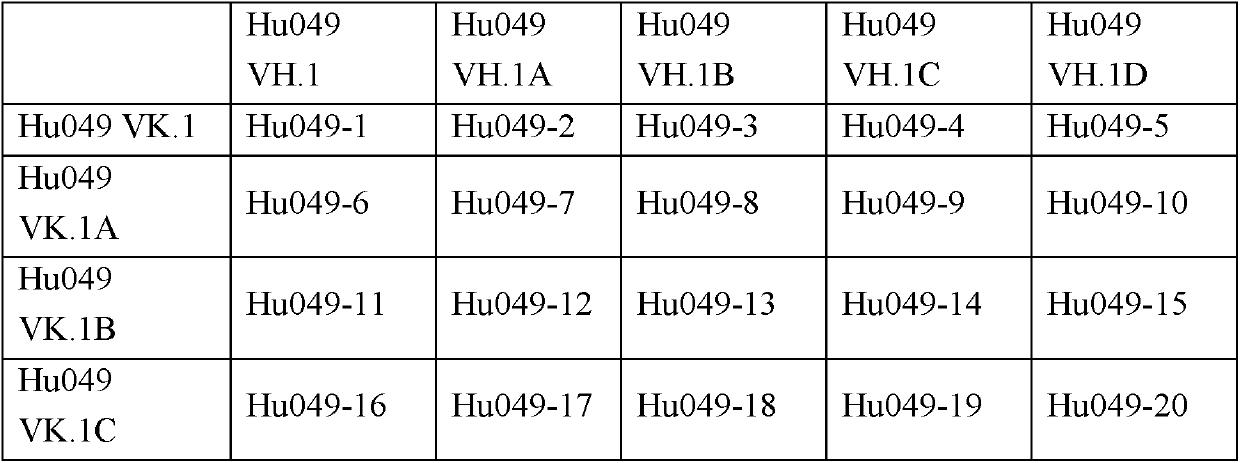
[0091] 2. Select human germline FR sequence
[0092] On the basis of the typical structure of the obtained murine antibody VH / VL CDR, the heavy and light chain variable region seq...
Embodiment 3
[0123] Example 3 In vivo pharmacokinetic detection of humanized anti-IL-17 antibody
[0124] Human IL-17 binds to and stimulates the mouse IL-17 receptor, leading to an increase and subsequent secretion of the KC(CXCL1) chemokine in male mice. Time and dose variation experiments were performed to identify the optimal dose of human IL-17 and the optimal timing of mouse KC induction (see Test Example 5). These experiments showed that a dose of 150 mg / kg of human IL-17 and a time period of 2 hours after IL-17 administration caused the highest levels of KC in mouse serum. The full-length antibody of the present invention was intravenously administered to mice at 3, 30, 300, or 3000 μg / kg 20 hours before subcutaneous injection of human IL-17. Two hours after human IL-17 administration, mice were sacrificed and KC levels were determined by ELISA using a commercial kit according to the manufacturer's instructions (Mouse CXCL1 / KC Quantikine ELISA Kit, R&D SYSTEM, #SMKC00B). An isoty...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com