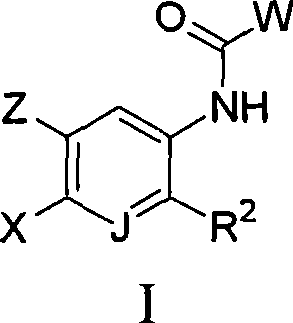
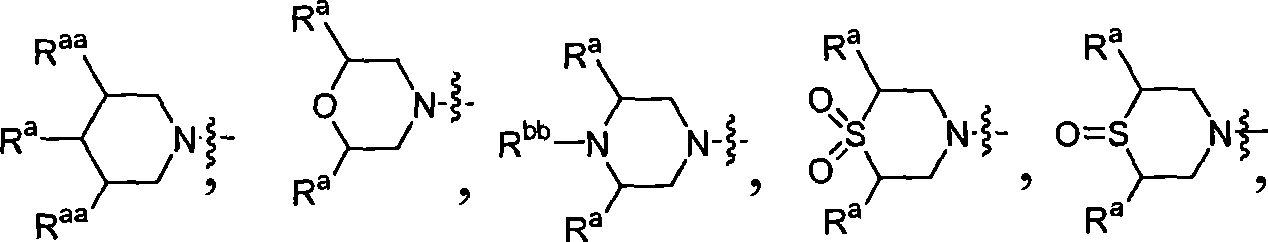
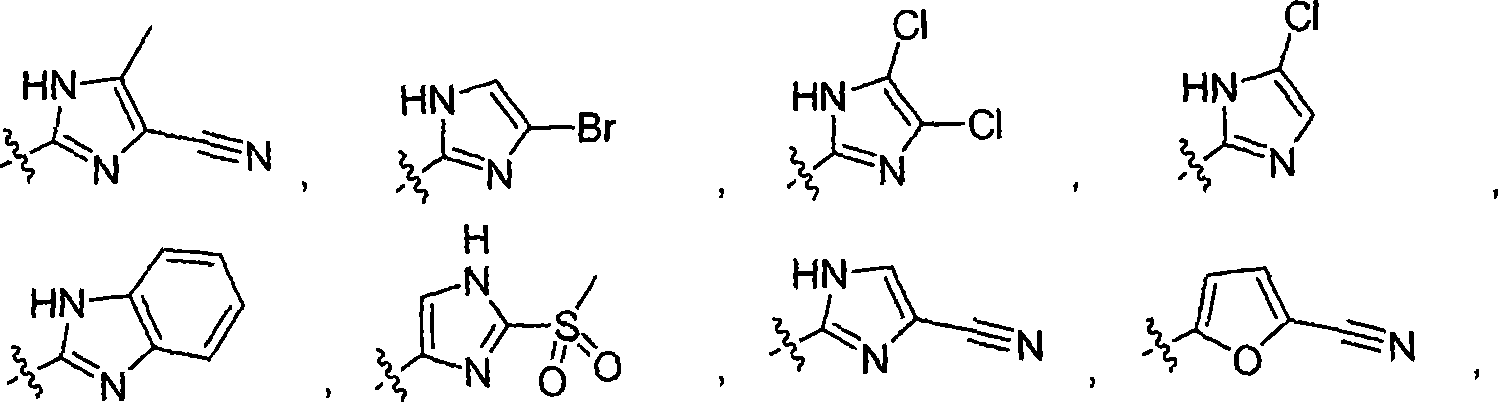
Inhibitors of C-FMS kinase
A CH3, solvate technology, applied in the field of compounds with c-fms kinase inhibitor function
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0315] N-[2-(4,4-Dimethyl-cyclohex-1-enyl)-4-(4-hydroxy-tetrahydro-pyran-4-yl)-phenyl]-5-cyano- 1H-imidazole-2-carboxamide
[0316]
[0317] a) 1-(2-trimethylsilyl-ethoxymethyl)-1H-imidazole-4-carbonitrile
[0318]
[0319] Imidazole-4-carbonitrile (0.50g, 5.2mmol) (Synthesis, 677, 2003), 2-(trimethylsilyl)ethoxymethyl chloride (SEMC1) (0.95mL, 5.3mmol), K 2 CO 3 (1.40g, 10.4mmol) and acetone (5mL) were added to the flask and stirred at room temperature for 10h. The mixture was diluted with EtOAc (20 mL), washed with water (20 mL), brine (20 mL), and the organic layer was washed over MgSO 4 dry. The crude product was eluted on a 20-g SPE cartridge (silica gel) with 30% EtOAc / hexanes to afford 0.80 g (70%) of the title compound as a colorless oil. Mass spectrometry (CI(CH 4 ), m / z): C10 h 17 N 3 Theoretical value of OSi, 224.1 (M+H), found value 224.1.
[0320] b) 2-bromo-1-(2-trimethylsilyl-ethoxymethyl)-1H-imidazole-4-carbonitrile
[0321]
[0322] To 1-(2-...
Embodiment 2
[0341] N-[4-[4-(2-Dimethylamino-ethoxy)-tetrahydro-pyran-4-yl]-2-(4,4-dimethyl-cyclohex-1-enyl )-phenyl]-5-cyano-1H-imidazole-2-carboxamide trifluoroacetate
[0342]
[0343]To N-[2-(4,4-dimethyl-cyclohex-1-enyl)-4-(4-hydroxy-tetrahydro-pyran-4-yl)-phenyl]-5-cyano -1H-imidazole-2-carboxamide (48.0 mg, 0.114 mmol) (the product of step (h) of Example 1) in 1 mL of DCM suspension was added 2-dimethylaminoethanol (0.114 mL, 1.14 mmol), TFA (0.130 mL, 1.17 mmol). The mixture was heated to 50 °C for 8 h. The mixture was concentrated and the title compound was purified by RP-HPLC on a C18 column with a linear gradient of 30-50% CH 3 CN / 0.1% TFA / H 2 O was eluted for 12 min to afford 14 mg (20%) of a white solid. 1 H-NMR (400MHz, CD 3 OD δ 8.21(d, J=8.6Hz, 1H), 7.91(s, 1H), 7.35(dd, J=8.6, 2.2Hz, 1H), 7.21(d, J=2.2Hz, 1H), 5.67(m , 1H), 3.83-3.66(m, 4H), 3.30-3.15(m, 4H), 2.76(s, 6H), 2.26-2.20(m, 2H), 2.12-1.94(m, 6H), 1.51(t , J=6.3Hz, 2H), 1.00 (s, 6H). Mass Spectrum (ESI...
Embodiment 3
[0345] {4-[4-[(5-cyano-1H-imidazole-2-carbonyl)-amino]-3-(4,4-dimethyl-cyclohex-1-enyl)-phenyl]-tetra Hydrogen-pyran-4-yloxy}-acetic acid
[0346]
[0347] To N-[2-(4,4-dimethyl-cyclohex-1-enyl)-4-(4-hydroxy-tetrahydro-pyran-4-yl)-phenyl]-5-cyano -1H-Imidazole-2-carboxamide (48.0 mg, 0.114 mmol) (the product of step (h) of Example 1) in 1 mL of DCM was added methyl glycolate (0.215 mL, 2.78 mmol), TFA (0.036 mL, 0.464 mmol). The mixture was stirred at room temperature for 8 h. The mixture was concentrated and the methyl ester of the title compound was eluted on a 10-g SPE cartridge with 50% EtOAc / hexanes. The obtained ester was dissolved in 1 mL MeOH, 2N KOH (0.30 mL, 0.60 mmol) was added, the mixture was stirred at room temperature for 8 h, the title compound was purified by RP-HPLC on a C18 column with a linear gradient of 30-60% CH 3 CN / 0.1%TFA / H 2 O was eluted for 12 min to afford 13 mg (30%) of a white solid. 1 H-NMR (400MHz, CD 3 OD δ 8.34(d, J=8.6Hz, 1H), 7.85...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com