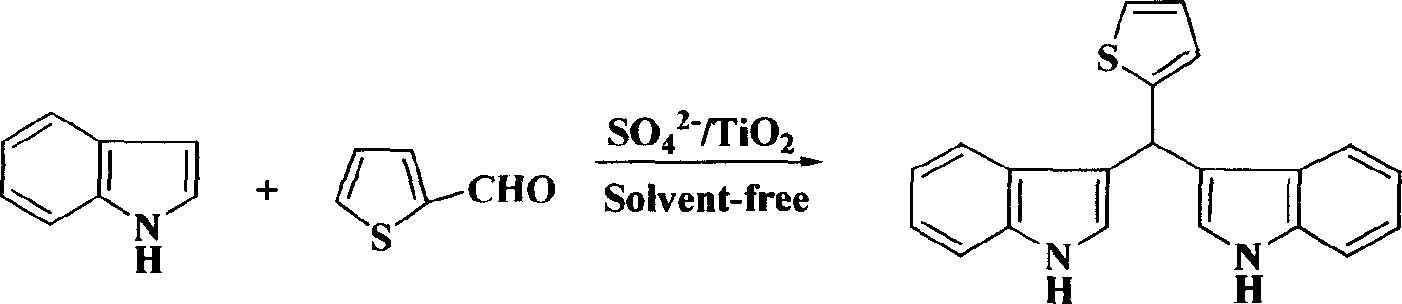Double indolyl derivative synthesizing process
A technology of indole alkyl and synthesis method, which is applied in the field of preparation of heterocyclic compounds, can solve problems such as not seen, and achieve the effects of no reduction in activity, avoiding pollution, and simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0016] Embodiment one: the synthesis of bis-indolylmethane
[0017] Take 1 mmol of formaldehyde and 2 mmol of indole in a grinder, add solid super acid SO 4 2- / TiO 2 Catalyst, the dosage is 50 mg, ground and reacted at 50°C for 0.5-1.5 hours, then washed with ethyl acetate, filtered, and the filtrate is concentrated to obtain a crude product, which is further purified to obtain bis-indolylmethane with higher purity.
Embodiment 2
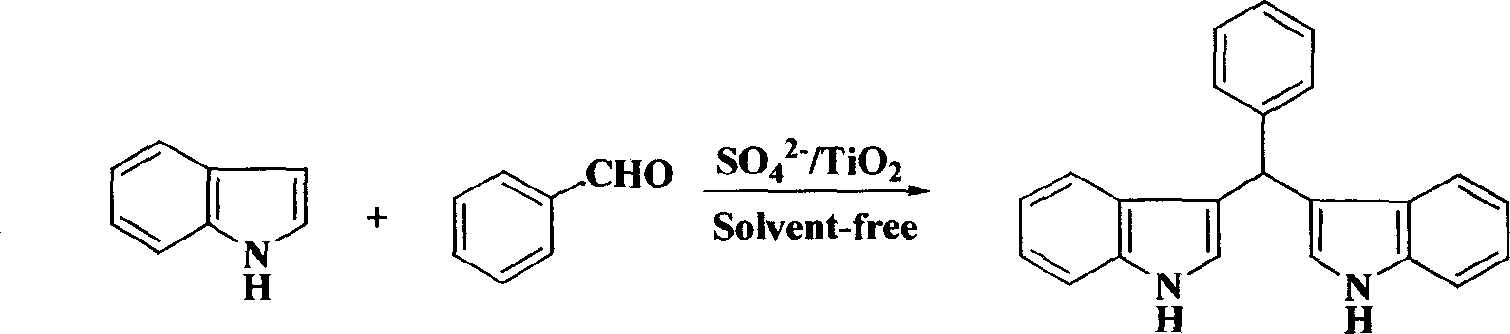
[0018] Embodiment two: the synthesis of bisindolephenyl methane
[0019] Take 1 mmol of benzaldehyde and 2 mmol of indole in a reaction flask, add 60 mg of solid superacid SO 4 2- / TiO 2 , placed in a grinder, ground evenly, heated up to about 50°C under an infrared lamp, after 0.5 hours, TLC detected that the reaction was complete, washed with ethyl acetate, filtered, and the filtrate was concentrated in vacuum to obtain a crude product, which was further purified to obtain a higher Purity bisindolephenylmethane.
[0020]
Embodiment 3
[0021] Embodiment three: the synthesis of bis-indoxythiophene methane
[0022] Take 1 mmol of thiophene-2-carbaldehyde and 2 mmol of indole in a reaction flask, add 45 mg of solid superacid SO 4 2- / TiO 2 , placed in a grinder, ground evenly, heated to about 50°C under an infrared lamp, after 1.5 hours, TLC detected that the reaction was complete, washed with ethyl acetate, filtered, and the filtrate was concentrated in vacuum to obtain a crude product, which was further purified to obtain a higher Purity bisindolephenylmethane.
[0023]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com