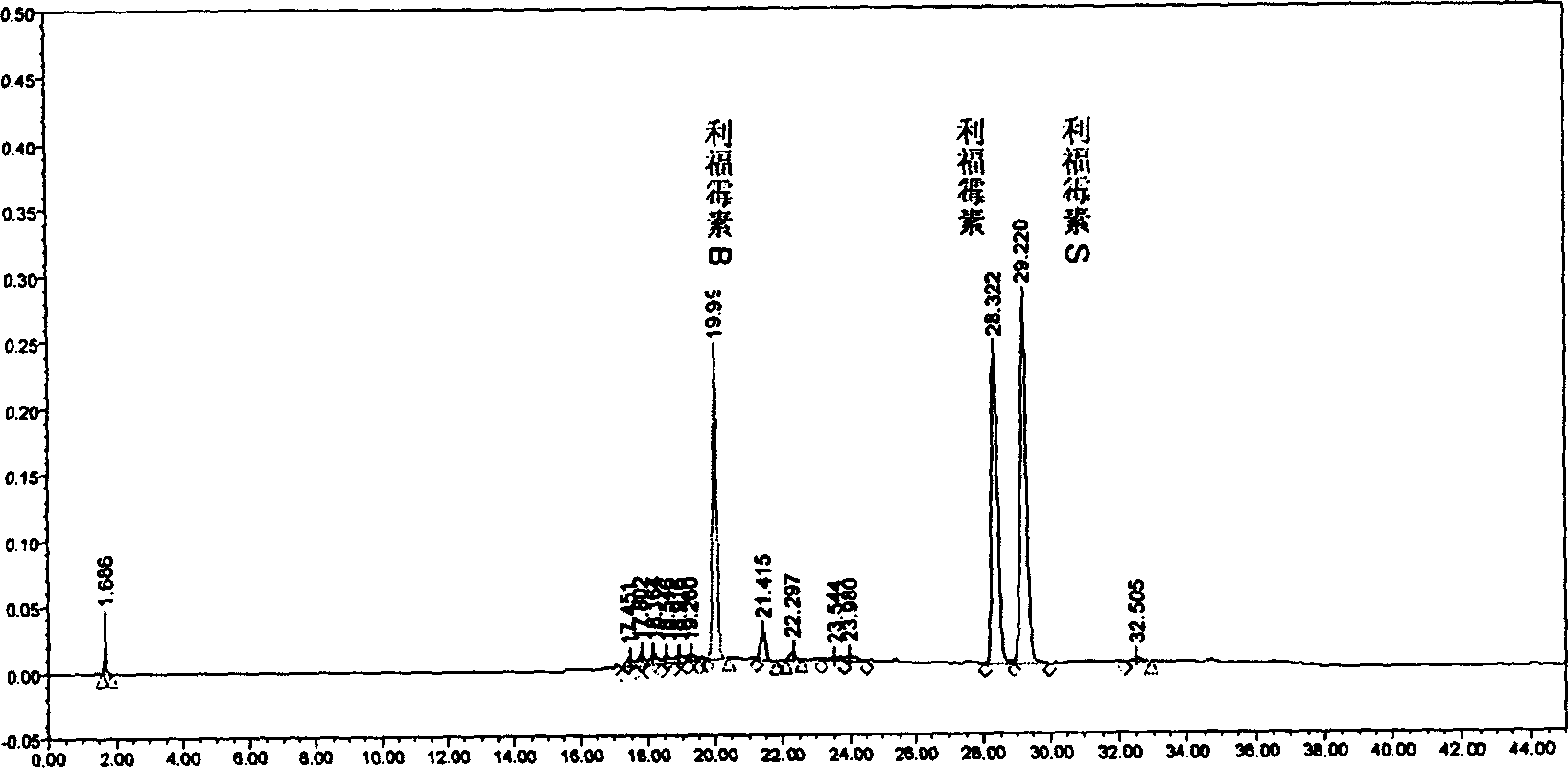
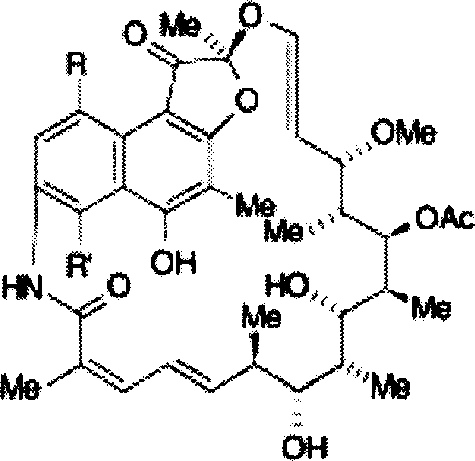
Intravenous injection stable concertrated clear solution containing rifamycin after dilution
A rifamycin and drug technology, applied in the field of concentrated clear solution, can solve the problems of affecting the curative effect, toxicity and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0040] The formulation examples of the stable clear solution of the active compound involved in the present invention are listed below to further explain the present invention, but are not limited by these examples.
[0041] (1) Rifamycin 25.0g
[0042] Propylene glycol 500.0ml
[0043] Dimethylformamide Add to 500.0ml
[0044] Total 1000.0ml
[0045] (2) Rifamycin 250.0g
[0046] Add propylene glycol to 1000.0ml
[0047] Total 1000.0ml
[0048] (3) Rifamycin 150.0g
[0049] Propylene glycol 800.0ml
[0050] Add to 200.0ml polyethylene glycol
[0051] Total 1000.0ml
[0052] (4) Rifamycin 120.0g
[0053] Absolute ethanol 500.0ml
[0054] Propylene glycol 300.0ml
[0055]Add polyethylene glycol to a total of 1000.0ml
[0056] (5) Rifamycin 120.0g
[0057] Absolute ethanol 400.0ml
[0058] ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com