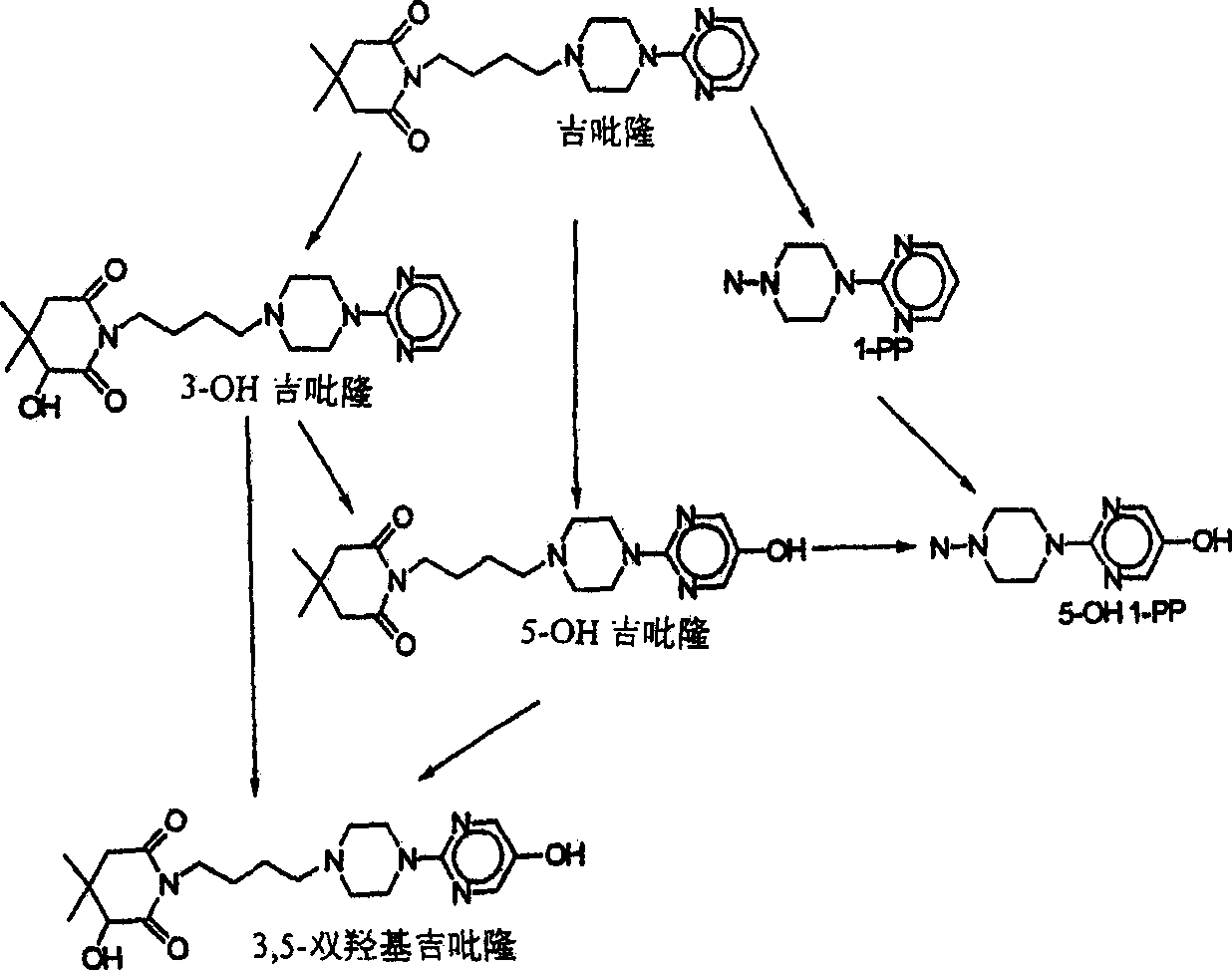
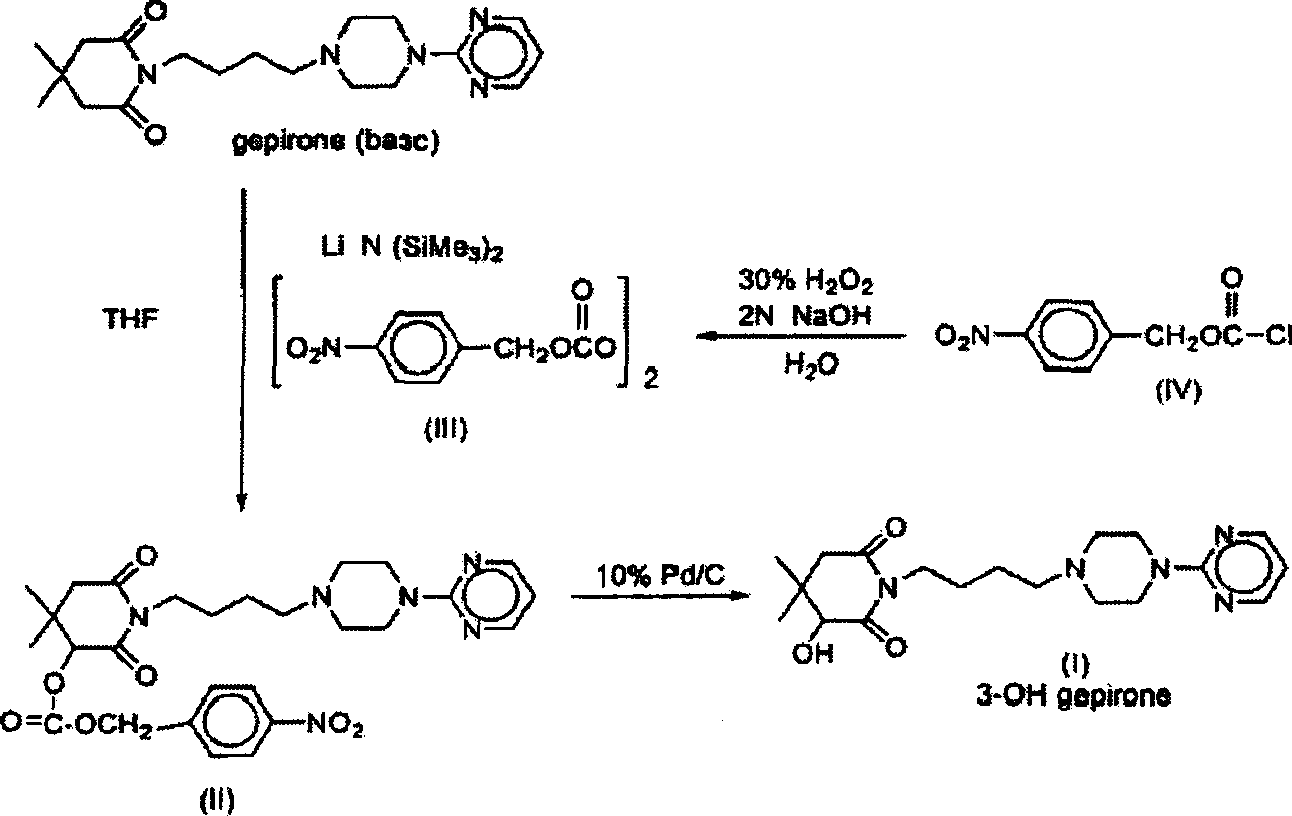
Use of bioactive metabolites of gepirone for the treatment of psychological disorders
A technology for mammals and psychological states, which can be used in drug combinations, medical preparations containing active ingredients, pharmaceutical formulations, etc., and can solve problems such as lack of biological activity.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0046] Embodiment 1: the preparation of 3-hydroxy gepirone (I)
[0047] A. Di-4-nitrobenzyl peroxydicarbonate (III)
[0048] Di-4-nitrobenzyl peroxydicarbonate can be prepared using a modification of a documented procedure (Strain et al., "Japan American Chemical Science", 1950, 72: 1254; incorporated herein by reference ). Thus, a cold solution of 4-nitrobenzyl chloroformate (10.11 g, 4.7 mmol) in acetone (20 mL) was added dropwise over a period of 30 minutes from 30% H 2 o 2 (2.7mL, 24mmol) and 2.35N NaOH (20mL, 47mmol) in a cold mixture. The mixture was stirred well for 15 minutes, then filtered, and the filter cake was rinsed first with water and then with hexane. The resulting wet solid was dissolved in dichloromethane, and the solution was dried over Na2SO4 and diluted with an equal amount of hexane. Concentration of the solution on a rotary dehydrator at 20°C resulted in the precipitation of crystals, which were filtered and rinsed with hexane, and dried in vacuo t...
Embodiment 2
[0057] Example 2: Comparison of 3-hydroxygepirone, gepirone metabolites and gepirone
[0058]
compound
Log ρ ow octanol-water partition coefficient
Crippen fraction
Viswanadhan fraction
Broto fraction
Gepirone
1.38±0.47
1.32±0.49
1.13±0.97
3-Hydroxygepirone
0.73±0.47
0.89±0.49
-0.23±1.11
[0059] In all methods, 3-hydroxygepirone has higher water solubility (low log ρ ow ) and have lower ester solubility compared with gepirone.
[0060] The short half-life of gepirone is attributed to its high oil solubility, which makes it more susceptible to first-pass degradation in the liver. Because 3-hydroxygepirone has little solubility in esters, its first-pass degradation profile results in a long half-life in plasma. In addition, the ester solubility range of 3-hydroxygepirone (approximately 5:1 to 8:1) is generally within the acceptable range for psychoactive drugs acting on brain recept...
Embodiment 3
[0061] Embodiment 3: 3-hydroxygepirone preparation
[0062] The 3-hydroxygepirone compositions and formulations of the present invention are designed to administer to mammals, preferably humans, an effective amount of an anxiolytic, antidepressant, psycho-altering 3-hydroxygepirone, or a pharmacologically acceptable form thereof. its salts. Effective formulations of about 0.01 to 40 mg / kg body weight are envisioned, with a preferred range of about 0.1-0.2 mg / kg body weight. For some central nervous disorders, it is recommended to take 15-90mg per day, preferably 30-60mg per day. (See US Patent 4,771,053 to Cott et al., incorporated herein by reference). The administration of the biologically active gepirone metabolite according to the present invention can be through injection, oral administration, buccal administration, rectal administration, external application, etc., but oral administration is preferred. For alleviating severe depression, the clinical dose range is less...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com