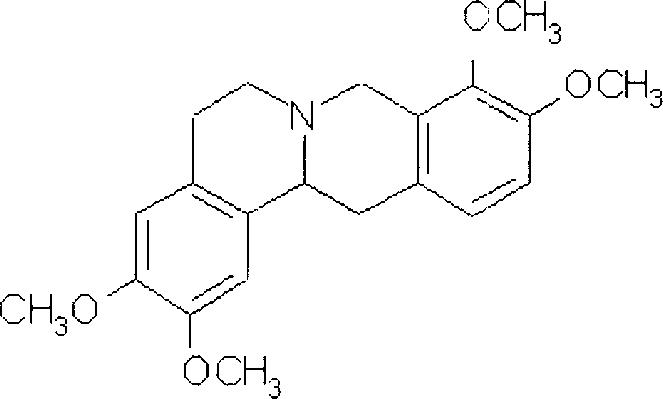
Slow release tablet of rotundine and production process
A technology of sustained-release preparations and sustained-release parts, which is applied in the field of new dosage forms - Rotundine sustained-release tablets, which can solve problems such as unsuitable for taking
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0016] Embodiment 1: the sustained-release preparation containing 105mg rotundine
[0017] Immediate release part: rotundine 45mg, polyvinylpyrrolidone 6mg, microcrystalline cellulose 40mg, lactose 20mg, magnesium stearate 1mg.
[0018] Mix the above-mentioned amount of rotundine, polyvinylpolypyrrolidone, microcrystalline cellulose, and lactose in equal amounts, add an appropriate amount of 10% povidone aqueous solution, and make a soft material, pass through a 12-20 mesh sieve Granulate, dry in an oven at 50-60°C, granulate, add the above-mentioned amount of magnesium stearate, mix well, analyze the content of the semi-finished product, and prepare the granules for later use.
[0019] Sustained-release part: 60mg of rotundine, 60mg of hydroxypropyl methylcellulose K4000, 30mg of L-tartaric acid, 2mg of magnesium stearate.
[0020] Mix the above amount of rotundine, hydroxypropyl methylcellulose K4000, and L-tartaric acid evenly, add 50% ethanol solution to make a soft mater...
Embodiment 2
[0022] Embodiment 2: the sustained release preparation of 120mg rotundine
[0023] Immediate release part: rotundine 30mg; polyvinylpyrrolidone 7mg, β-cyclodextrin 100mg, magnesium stearate 1mg.
[0024] Production process: Weigh 30 mg of rotundine, add ethanol to dissolve, add dropwise to 100 mg of β-cyclodextrin (β-cyclodextrin: water = 20:80w / v) solution heated to 70-80°C, and stir for 2 hours , filter the precipitate, and dry it in a 50-60°C oven to obtain a mixture of rottondine and β-cyclodextrin. Mix with the above-mentioned amount of polyvinylpolypyrrolidone and process according to the method in Example 1.
[0025] Sustained-release components: rotundine 90mg, hydroxypropyl methylcellulose K 4000 90mg, L-tartaric acid 45mg, magnesium stearate 2mg are processed by the method described in Example 1 to obtain the sustained-release preparation of 120mg rotundine / tablet.
Embodiment 3
[0026] Example 3: Sustained release formulation of rotundine 210 mg.
[0027] Immediate-release components: 90 mg of rotundine, 12 mg of polyvinylpyrrolidone, 80 mg of microcrystalline cellulose, 40 mg of lactose, and 2 mg of magnesium stearate, processed according to the method of Example 1 to obtain immediate-release component granules.
[0028] Sustained release components: rotundine 120mg, hydroxypropyl methylcellulose K 4000 120mg, L-tartaric acid 60mg, magnesium stearate 4mg, according to embodiment 1 sustained-release component processing and tabletting processing method, obtain the sustained-release preparation of 210mg rotundine / tablet.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com