Patents
Literature
44 results about "Analeptic" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
An analeptic, in medicine, is a central nervous system stimulant. The term analeptic typically refers to respiratory analeptics (for example, doxapram). Analeptics are central nervous system stimulants that include a wide variety of medications used to treat depression, attention deficit hyperactivity disorder (ADHD), and respiratory depression. Analeptics can also be used as convulsants, with low doses causing patients to experience heightened awareness, restlessness and rapid breathing. The primary medical use of these drugs is as an anesthetic recovery tool or to treat emergency respiratory depression. Other drugs of this category are prethcamide, pentylenetetrazole, and nikethamide. Nikethamide is now withdrawn due to risk of convulsions. Analeptics have recently been used to better understand the treatment of a barbiturate overdose. Through the use of agents researchers were able to treat obtundation and respiratory depression.
Nutrigenomics methods and compositions
The present invention provides a proprietary compositions and systems to modulate genetic and metabolomic contributing factors affecting disease diagnosis, stratification, and prognosis, as well as the metabolism, efficacy and / or toxicity associated with specific vitamins, minerals, herbal supplements, homeopathic ingredients, and other ingredients for the purposes of customizing a subject's nutritional supplement formulation to optimize specific health outcomes. Specific to this invention the utilization of certain known polymorphic genes associated with Substance Use Disorder (SUD) are analyzed to target certain genetic anomalies that lead to a high risk and predisposition to SUD. The genotypic patterns are then utilized to provide certain nutritional customized solutions especially related to the attenuation of aberrant abuse of physician prescribed narcotic pain medication across all pain conditions. A priority GENOPROFILE is measured and directs the customization of a subsequent nutraceutical to act as a therapeutic modality. Specifically the treatment includes slow attenuation of the pain medication by incorporating orals (shakes, liquid beverages, pills, tablets, troche, ointments etc.), Intramuscular, Intravenous, intra-rectal and any form necessary to deliver a sufficient amount of an anti-craving and anti-stress nutraceutical. Moreover, the invention includes examples of novel analgesic ointments coupling Synaptamine and such analgesic and other anesthetic compounds including but not limited to Gabapentin, Ketamine, Baclofen, Ketoprofen, Amitriptyline, Lidocaine, Cyclobenzapine, Diclofenac, Menthol, Camphor and Capsaicin. The GENOPROFILE will be used to determine pain sensitivity Intolerance.
Owner:BLUM KENNETH +3
Synergistic combinations including N-acylated 4-hydroxyphenylamine derivatives
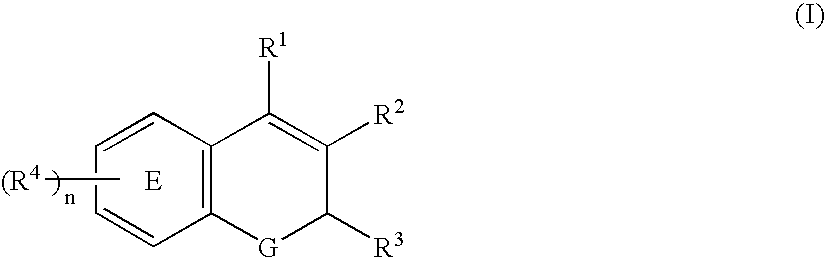
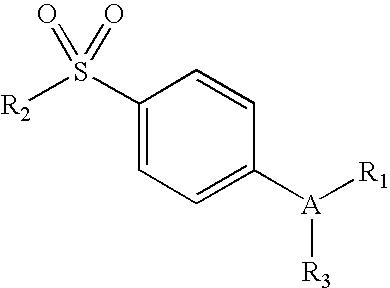
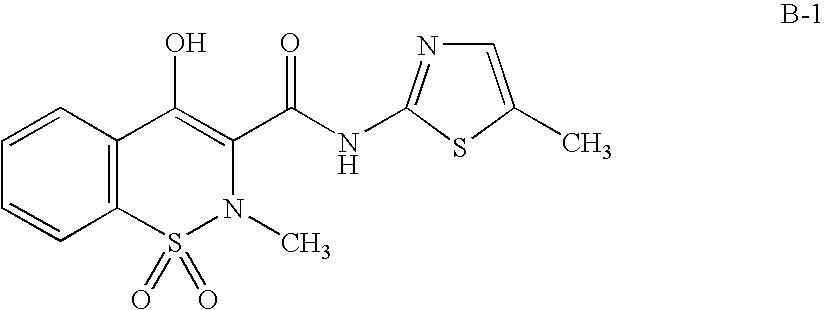
The present invention relates to pharmaceutical combinations of opioid and non-opioid analgesics in an intimate admixture with an analgesic from a series of N-acylated 4-hydroxyphenylamine derivatives, linked via an alkylene bridge to the nitrogen atom of a 1,2-benzisothiazol-3(2H)-one 1,1-dioxide group and methods for their use to alleviate pain in mammals. The analgesic combinations exhibit enhanced analgesic potency, do not suppress blood coagulation, and have little hepatotoxic effect.
Owner:BOARD OF SUPERVISORS OF LOUISIANA STATE UNIV & AGRI & MECHANICAL COLLEGE +1
Compositions of a cyclooxygenase-2 selective inhibitor and a central nervous system stimulant for the treatment of central nervous system damage
The present invention provides compositions and methods for the treatment of central nervous system damage in a subject. More particularly, the invention provides a combination therapy for the treatment of a central nervous system ischemic condition or a central nervous system traumatic injury comprising the administration to a subject of a central nervous system stimulant in combination with a cyclooxygenase-2 selective inhibitor.
Owner:PHARMACIA CORP
Abuse resistant opioid dosage form
InactiveUS20060058331A1Inhibit and discourage abuseInhibit and discourage of dosage formBiocideAnimal repellantsOpioid antagonistN-methyl-D-aspartate Receptor Antagonists
The present invention pertains to a pharmaceutical dosage form comprising an opioid analgesic and a nontoxic N-methyl-D-aspartate receptor antagonist wherein the pharmaceutical dosage form is substantially free of an opioid antagonist The nontoxic N-methyl-D-aspartate receptor antagonist is present in an opioid euphoria-inhibiting amount to prevent or discourage abuse.
Owner:ENDO PHARMA INC
Analeptic rapid testing biological chip and testing method
The present invention provides relative reagent and inspection chip. The inspection method utilizes specificity integration character of excitant receptor protein to excitant molecule to fix relative receptor on surface of solid phase carrier so that excitant composition in test sample can be inspected out for quick screening of tested objects through competition action of excitant molecule and labelled excitant molecule on the receptor.
Owner:INST OF MATERIA MEDICA AN INST OF THE CHINESE ACAD OF MEDICAL SCI +1
Methods and compositions particularly for treatment of attention deficit disorder
ActiveUS9974752B2Act quicklyLong duration of actionPowder deliveryOrganic active ingredientsImmediate releasePharmaceutical drug
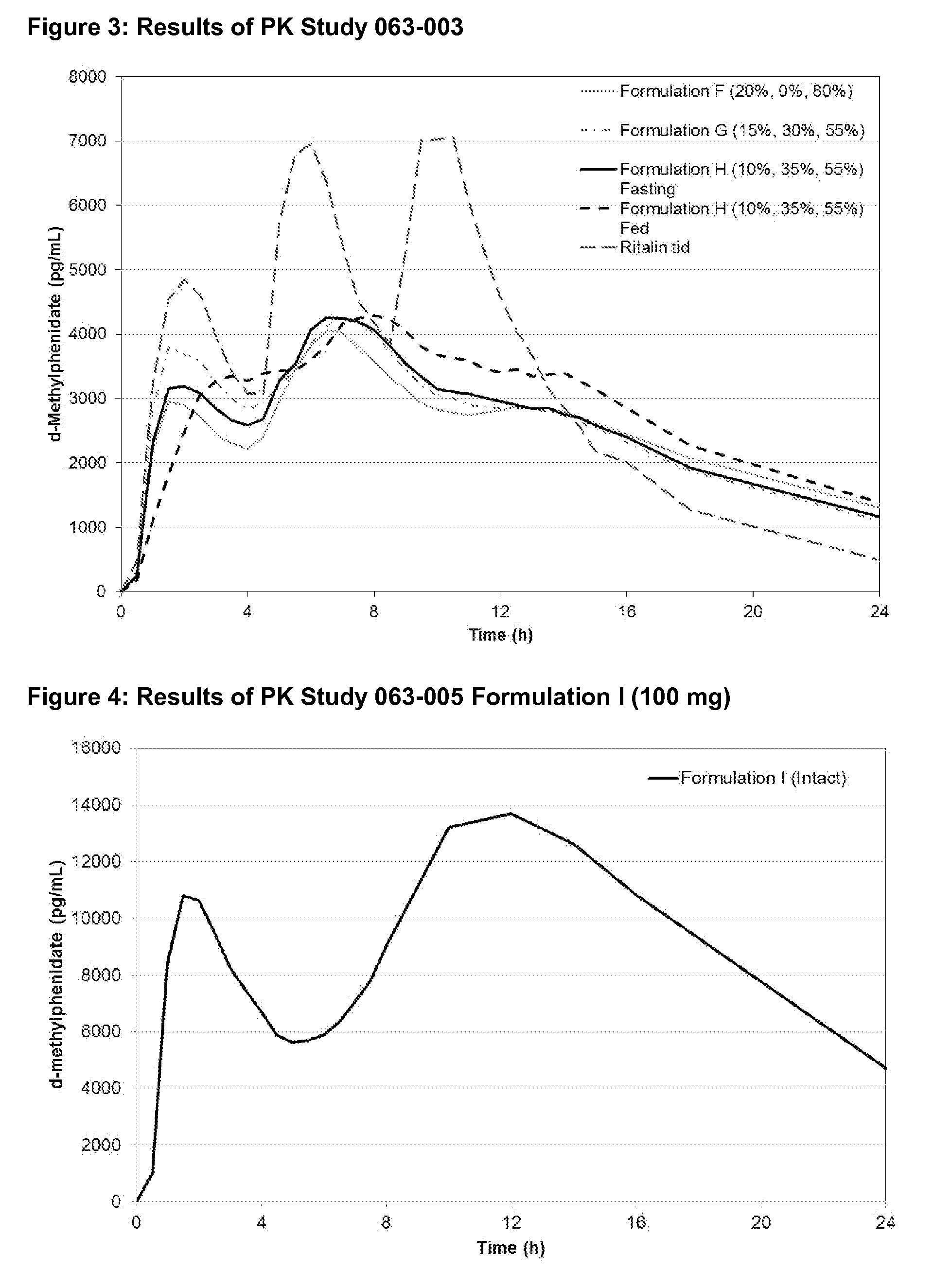
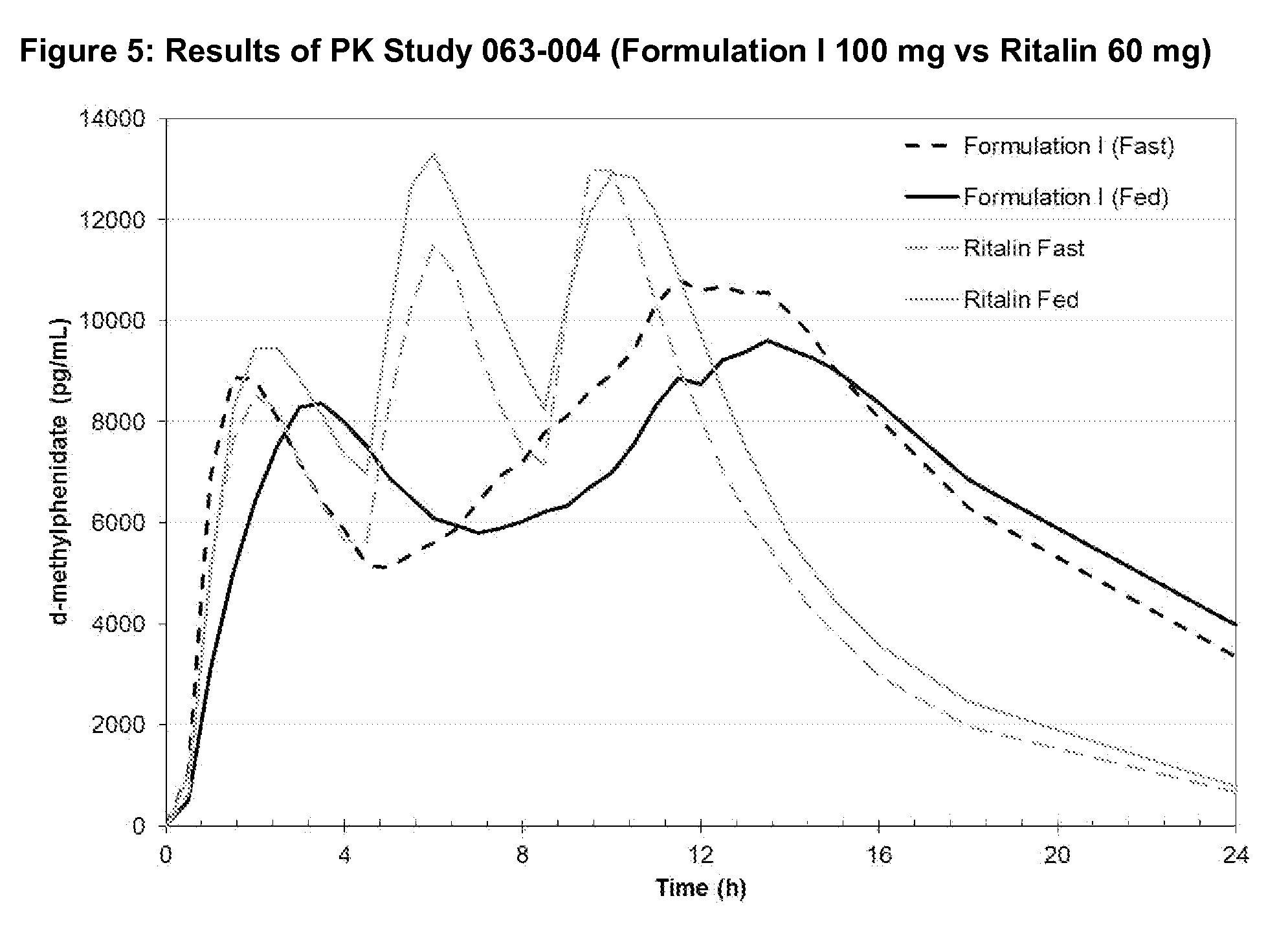
There is described, inter alia, a coated bead comprising: (a) a granule; (b) a first layer coated over the granule, the first layer comprising a first amount of an active pharmaceutical ingredient comprising a central nervous system stimulant; and (c) a second layer coated over the first layer, the second layer being present in an amount sufficient to substantially delay release of the active pharmaceutical ingredient in the first layer until after the coated bead reaches a distal intestine portion of a subject to whom the coated bead is administered; and (d) the third layer coated over the second layer, the third layer comprising a second amount of the active pharmaceutical ingredient, the third layer being configured to permit substantially immediate release of the active pharmaceutical ingredient comprised therein. Embodiments related to a solid oral pharmaceutical composition are also described.
Owner:PURDUE PHARMA LP
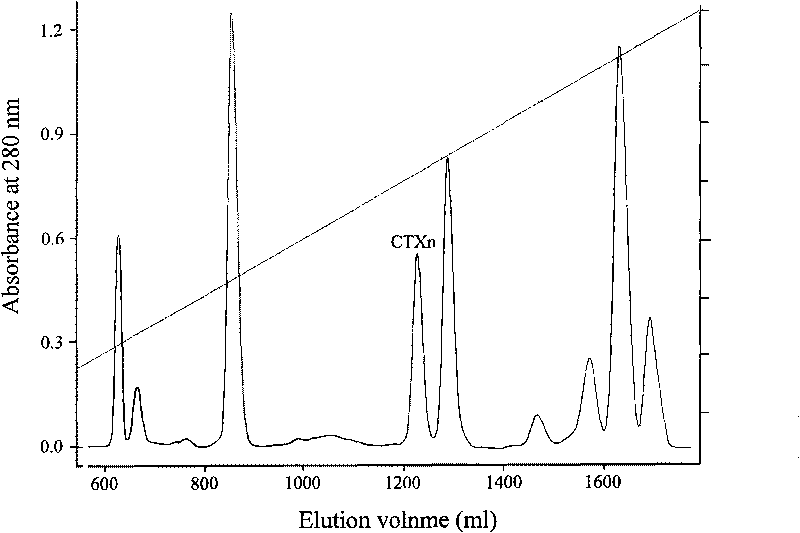
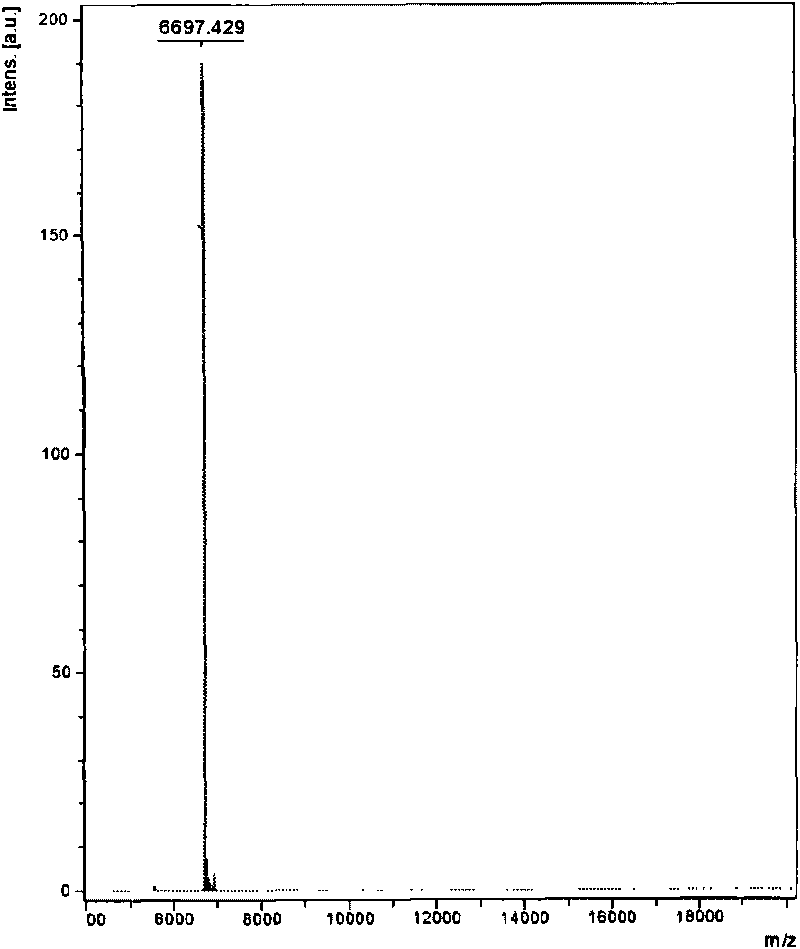
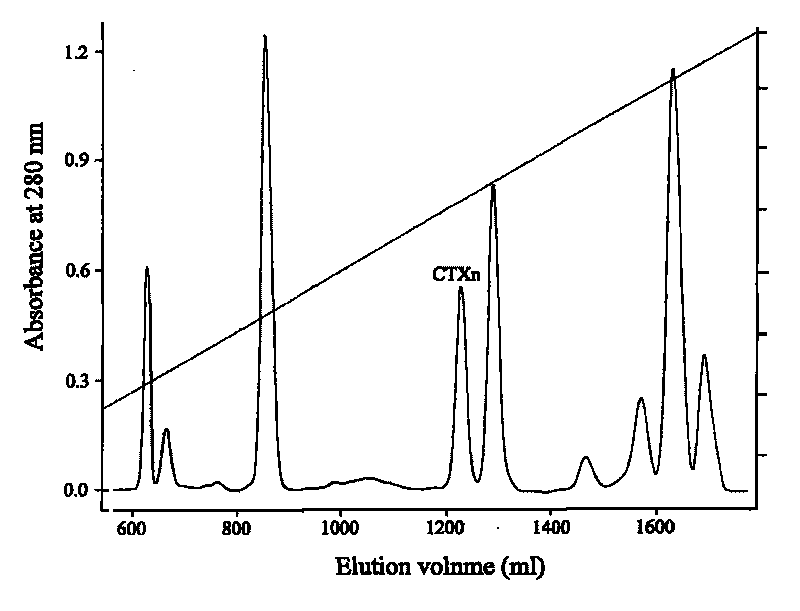
Venin-sourced demulcent CTXn, and purification method and applications thereof
InactiveCN101717441ARaise the response thresholdGood analgesic effectPeptide/protein ingredientsAntipyreticCobra venomVirulent characteristics
The invention relates to a venin-sourced demulcent CTXn, and a purification method and applications thereof. The invention is characterized in that (1) the molecular weight of the CTXn is 6697.429 Da; (2) and the amino acid sequence of the CTXn is described as the sequence list SEQ ID No.1. The preparation method comprises the following steps of: using a Zhoushan cobra venom sample; carrying out the CTXn separation and purification on the Zhoushan cobra venom sample; primary-screening to select a component III for next step of ion exchange; carrying out the ion exchange on the component III through a quick-purification technique exploitation system by using a filler to obtain a single-virulence polypeptide CTXn, desalting with a G-50 prepacked column, and freeze-drying; and measuring the molecular weight and the amino acid sequence of the CTXn. The CTXn of the invention has favorable demulcent function on somatalgia and visceralgia caused by nociceptive stimulus as well as pains caused by addiction to morphine, has the characteristics of obvious drug effect and low toxic or side effect, is beneficial to large-scale production, and has wide application prospects in the aspect of analgesic development.
Owner:广州医学院
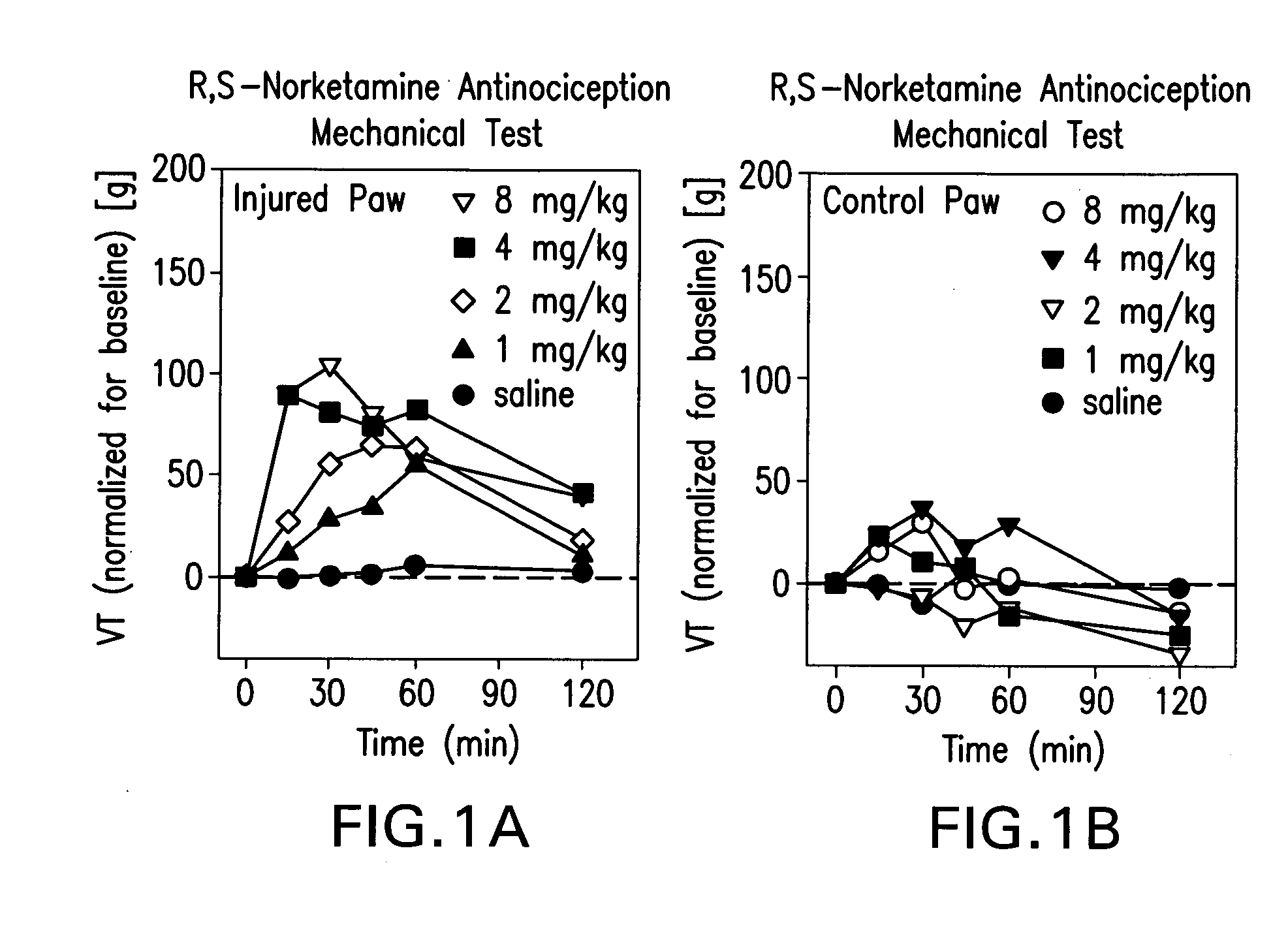
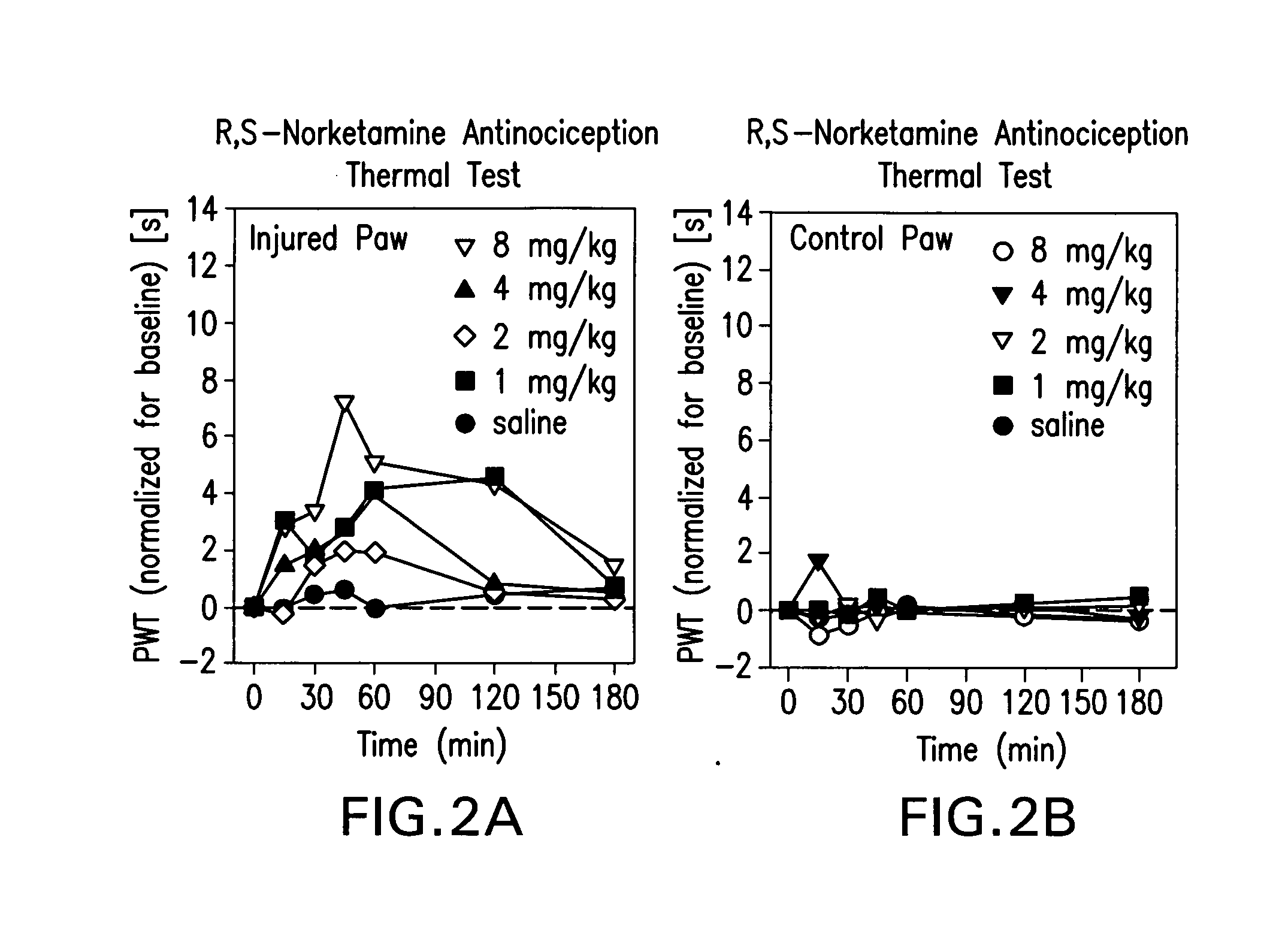
Synergistic combinations of norketamine and opioid analgesics
InactiveUS20080132531A1Rapid introductionExcellent dose-to-effect administration of drugBiocideNervous disorderHeadachesAnalgesic agents
The present invention relates to methods of alleviating pain with the administration of norketamine with a narcotic. More particularly, the invention provides a method of alleviating pain through the administration of a dose of norketamine, which, if administered alone would provide sub-optimal analgesic relief, yet provides analgesic relief when combined with a narcotic. In some embodiments, the combination of norketamine with a narcotic, further allows for the administration of a dose narcotic, which would be sub-optimal if used alone, but provides adequate pain relief in combination with norketamine. The invention relates to self-management of pain on an outpatient basis comprising administering via conventional routes, including transdermal, nasal, rectal, oral, transmucosal, intravenous, intramuscular, and other routes, one or more doses of norketamine / opioid compositions effective to alleviate pain to a subject suffering from pain. Uses of norketamine / opioid compositions would also apply, to treating headaches, drug abuse, mood and anxiety disorders, as well as other, neuropsychiatric disorders, both motoric and cognitive, such as Alzheimer's disease, Parkinson's syndrome, which are thought to be caused by neurodegeneration.
Owner:UNIV OF KENTUCKY RES FOUND
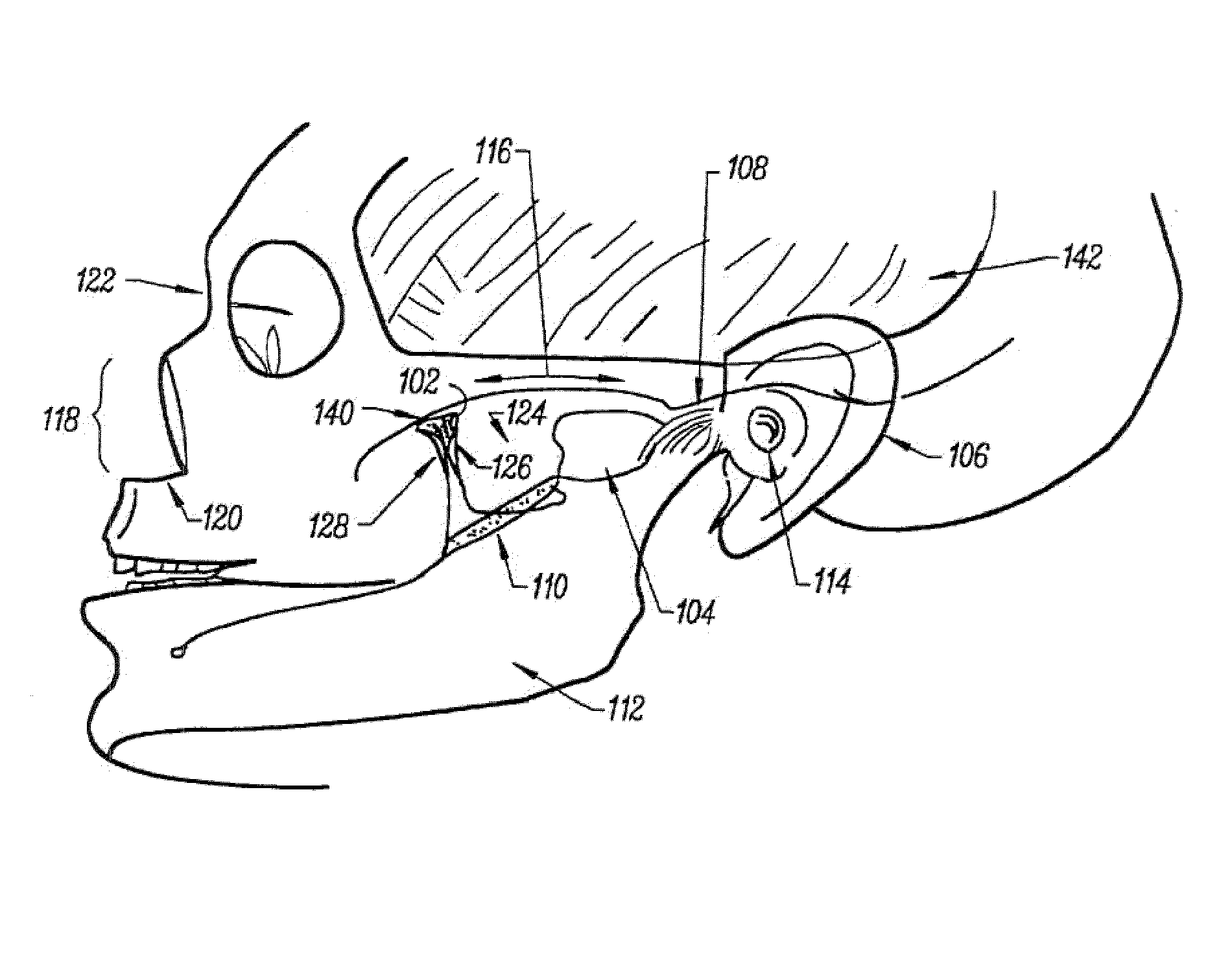
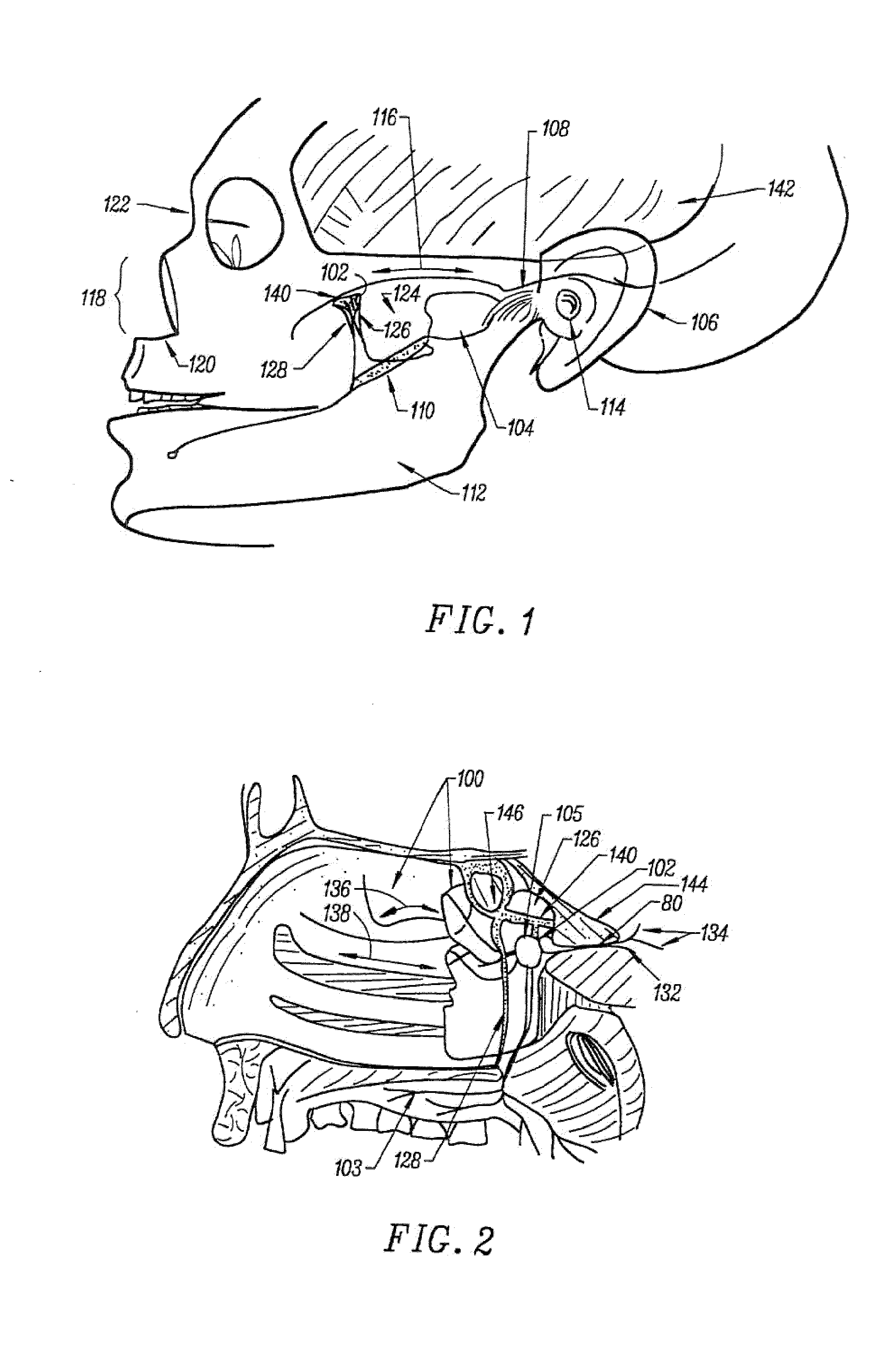
Stimulation method for the sphenopalatine ganglia, sphenopalatine nerve, or vidian nerve for treatment of medical conditions
A method is provided for the suppression or prevention of pain, movement disorders, epilepsy, cerebrovascular diseases, autoimmune diseases, sleep disorders, autonomic disorders, urinary bladder disorders, abnormal metabolic states, disorders of the muscular system, and neuropsychiatric disorders in a patient. The method comprises positioning at least one electrode on or proximate to at least one of the patient's sphenopalatine ganglia (“SPG”), sphenopalatine nerves (“SPN”), or vidian nerves (“VN”), and activating the at least one electrode to apply an electrical signal to at least one of the SPG, SPN, or VN. In a further embodiment of the invention used to treat the same conditions, the electrode used is capable of dispensing a medication solution or analgesic which is applied via an electrode to at least one of the SPG, SPN, or VN. A method is also provided for surgically implanting an electrode on or proximate to at least one of the SPG, SPN, or VN of a patient.
Owner:ANSARINIA MEHDI M
Ibuprofen solutions for capsule-filling and capsule preparations
InactiveCN1471389AAvoid bitternessQuick-actingNervous disorderHydroxy compound active ingredientsPh controlStimulant
Capsule preparations are produced by using solutions for capsule-filling which contain ibuprofen, polyethylene glycol, water and terpenes (menthol, limonene, borneol, dl-camphor, mentha oil, etc.) optionally together with one or more drugs selected from among antipyretic analgesics, antihistamines, antitussives, expectorants, sympathetic stimulant, analeptics, hypnotic sedatives and anti-inflammatory agents, solubilizers, thickeners, pH controlling agents, colorants, etc. Thus, it is attempted to relieve the bitterness of ibuprofen and establish an immediate action at the same time.
Owner:KOWA CO LTD
Slow release tablet of rotundine and production process
Owner:INST OF PHARMACOLOGY & TOXICOLOGY ACAD OF MILITARY MEDICAL SCI P L A
Methods of treating pain
The invention relates to methods for treating pain disorders including neuropathic and inflammatory pain and to methods to reduce or eliminate nociceptive tolerance induced by opiate analgesic use by administering an agent that suppresses or blocks S1P biological activity.
Owner:SAINT LOUIS UNIVERSITY
Beta analeptic clenbuterol immune affinity column, its preparing method and use
The invention discloses a method to distill beta2-excitant immune avidity column that could be used to distill beta2-exitant in forage and cattle. It includes beta2-excitant specificity antibody, coupling with beta2-exitant specificity antibody Sepharose4B, and plastic column, buffering liquid, eluate and eluent. The invention is convenient to use, has high specificity, and could rapidly distil beta2-exitant in forage and cattle.
Owner:北京中一茶文化发展有限公司
Methods and Compositions Particularly for Treatment of Attention Deficit Disorder
ActiveUS20180235895A1Act quicklyLong duration of actionPowder deliveryOrganic active ingredientsImmediate releasePharmaceutical drug
There is described, inter alia, a coated bead comprising: (a) a granule; (b) a first layer coated over the granule, the first layer comprising a first amount of an active pharmaceutical ingredient comprising a central nervous system stimulant; and (c) a second layer coated over the first layer, the second layer being present in an amount sufficient to substantially delay release of the active pharmaceutical ingredient in the first layer until after the coated bead reaches a distal intestine portion of a subject to whom the coated bead is administered; and (d) the third layer coated over the second layer, the third layer comprising a second amount of the active pharmaceutical ingredient, the third layer being configured to permit substantially immediate release of the active pharmaceutical ingredient comprised therein. Embodiments related to a solid oral pharmaceutical composition are also described.
Owner:PURDUE PHARMA LP
Methods and compositions particularly for treatment of attention deficit disorder
ActiveUS20160120819A1Rapid on set of actionLong duration of actionBiocidePowder deliveryNervous systemDrug
There is described, inter alia, a coated bead comprising: (a) a granule; (b) a first layer coated over the granule, the first layer comprising a first amount of an active pharmaceutical ingredient comprising a central nervous system stimulant; and (c) a second layer coated over the first layer, the second layer being present in an amount sufficient to substantially delay release of the active pharmaceutical ingredient in the first layer until after the coated bead reaches a distal intestine portion of a subject to whom the coated bead is administered; and (d) the third layer coated over the second layer, the third layer comprising a second amount of the active pharmaceutical ingredient, the third layer being configured to permit substantially immediate release of the active pharmaceutical ingredient comprised therein. Embodiments related to a solid oral pharmaceutical composition are also described.
Owner:PURDUE PHARMA LP
Methods and compositions for treating pain
InactiveUS20170143681A1Shorten the progressReduce severityOrganic active ingredientsDopamine receptor D1Chronic pain
The invention features combinations of dopaminergic agents and analgesic agents useful for treating pain. In particular, the combinations feature a low ratio of dopaminergic agent to analgesic agent. The dopaminergic agent can be an agonist of the dopamine receptor D1-like family or the dopamine receptor D2-like family. Such combinations potentiate analgesia to 1) alleviate acute pain, 2) prevent the transition from acute pain to chronic pain, and 3) manage chronic pain.
Owner:APKARIAN TECH
Composition comprising an edible acid or its acidic salt and the use thereof
This invention relates to a drug containing edible acid and / or acidic salt as active agent to treat and alleviate hypersensitivity diseases by lowering the humor pH and method; to improve individual hypersensitivity diseases by uses of health care food, which are made from said edible acid and / or acidic salt, or the acidic fruits containing thereof, or their products; foods lowering the risk of hypersensitivity and their preparation methods; drug to lower the humor acidity and to treat or alleviate disease caused by insects bite toxin; that also to be drug for cold or virus infection; drug for inflammation; drug for analgesic; and drug for thrombus disease; method lowers the hypersensitivity risk of clothing or grove and their products.
Owner:SHIAO SHIN JEN
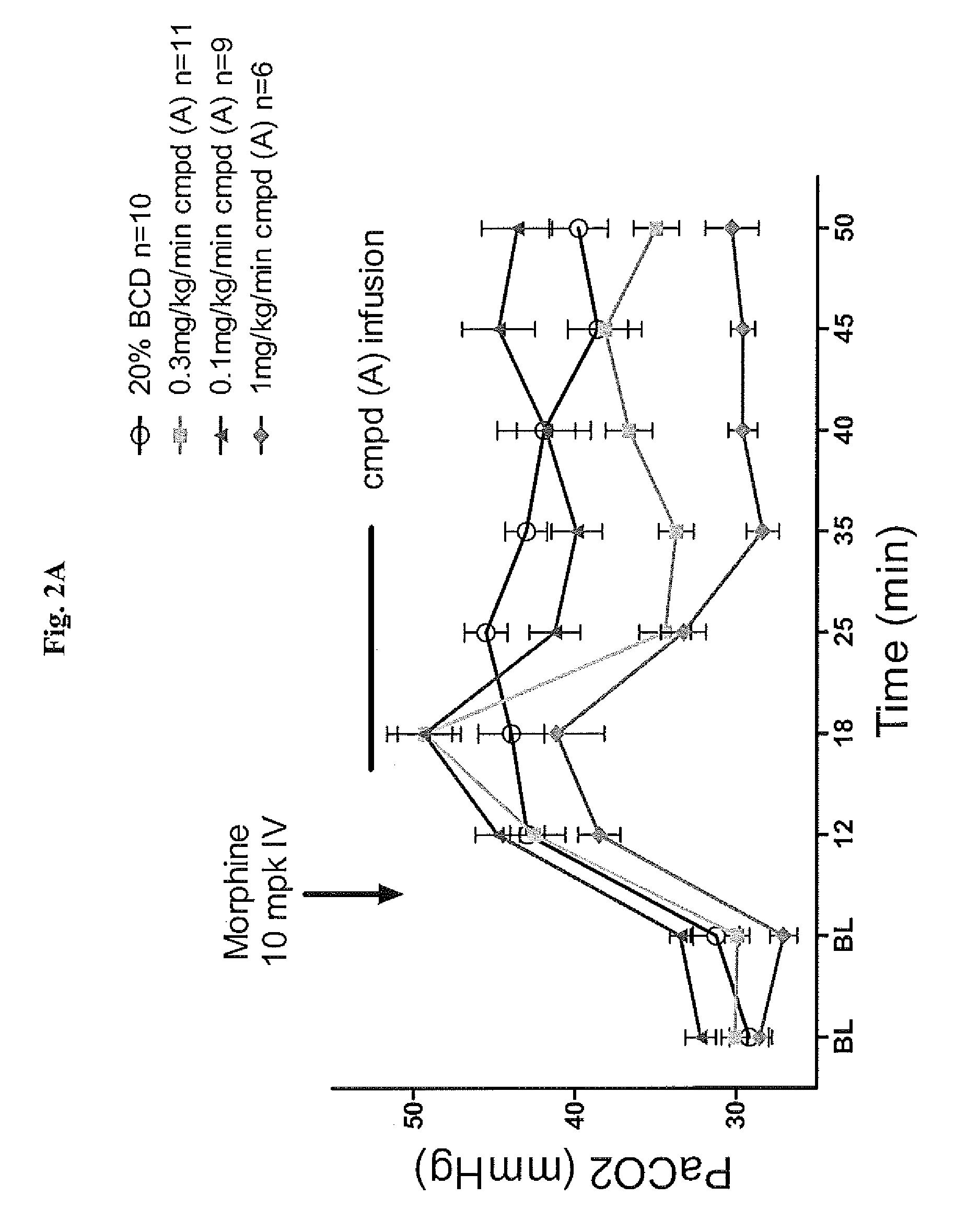
Compounds as respiratory stimulants for treatment of breathing control disorders or diseases
The present invention includes a composition comprising a compound, such as a 2,4,6-triamino-1,3,5-triazine, 2,4,6-triaminopyrimidine, 2,4-diamino-6,7-dihydro-5H-pyrrolo[2,3-d]pyrimidine or 2,4-diamino-7H-pyrrolo[2,3-d]pyrimidine, that is useful in the treatment of breathing control diseases or disorders in a subject in need thereof. The present invention also includes a method of treating a respiratory disease or disorder in a subject in need thereof, comprising administering to the subject a therapeutically effective amount of a pharmaceutical composition of the invention. The present invention further includes a method of preventing destabilizing or stabilizing breathing rhythm in a subject in need thereof, comprising administering to the subject a therapeutically effective amount of a pharmaceutical composition of the invention.
Owner:ENALARE THERAPEUTICS LLC
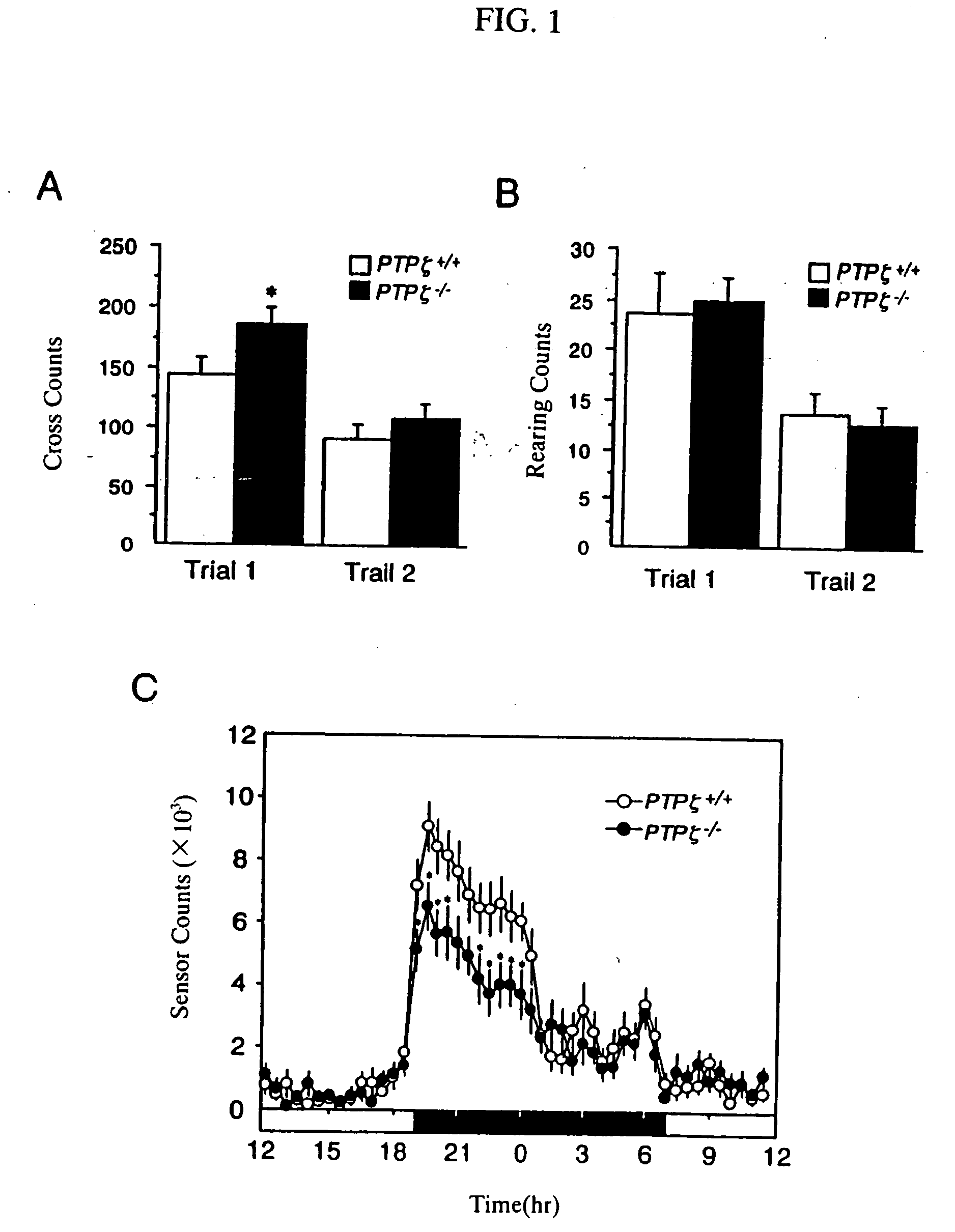
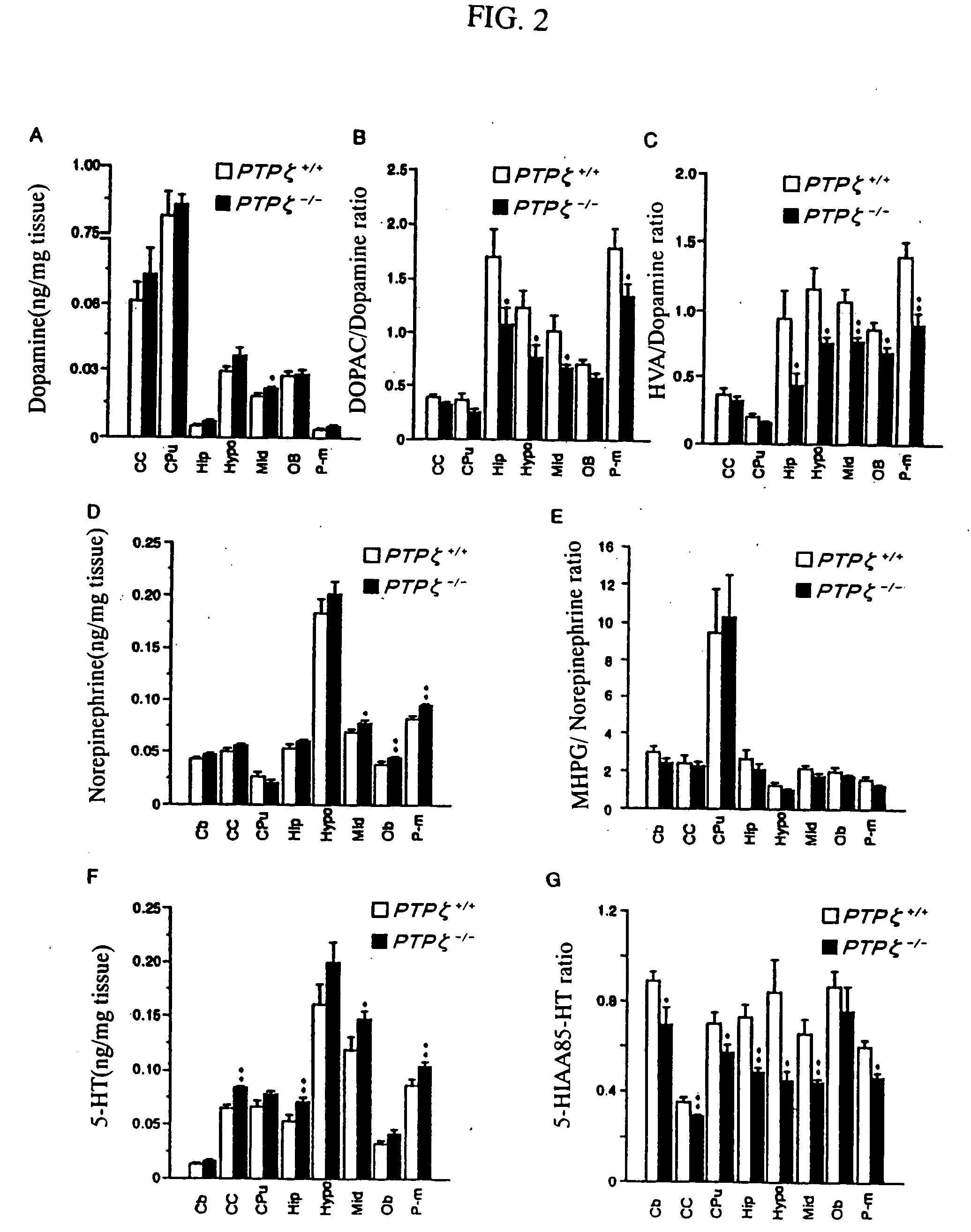
Method for screening PTPzeta activity promoter or inhibitor
An object of the present invention is to provide a remedy for dysfunction of central monoamine pathway, a method for screening a PTPζ inhibitor or activator, which is useful as a remedy for gastric ulcer caused by Helicobacter pylori or pleiotrophin which is a heparin-binding secretory protein, and a non-human model animal being hyposensitive to a stimulant drug, VacA which is a toxin of Helicobacter pylori, or pleiotrophin by utilizing the physiological function of PTPζ. After administering a subject material to PTPζ knockout mice and wild-type mice, PTPζ activity in the PTPζ knockout mice and the wild-type mice is compared and evaluated to screen a PTPζ inhibitor or activator. Examples of the comparison and the evaluation of the PTPζ activity include the comparison and the evaluation of the function of central monoamine pathway such as changes in the level of central monoamine metabolism, sensitivity to a stimulant drug, the presence of dysfunction of mesolimbic dopamine pathway, level of acclimation to new circumstances, or stress-responsiveness, and the comparison and the evaluation of the level of binding to VacA, a toxin of Helicobacter pylori, or pleiotrophin.
Owner:JAPAN SCI & TECH CORP
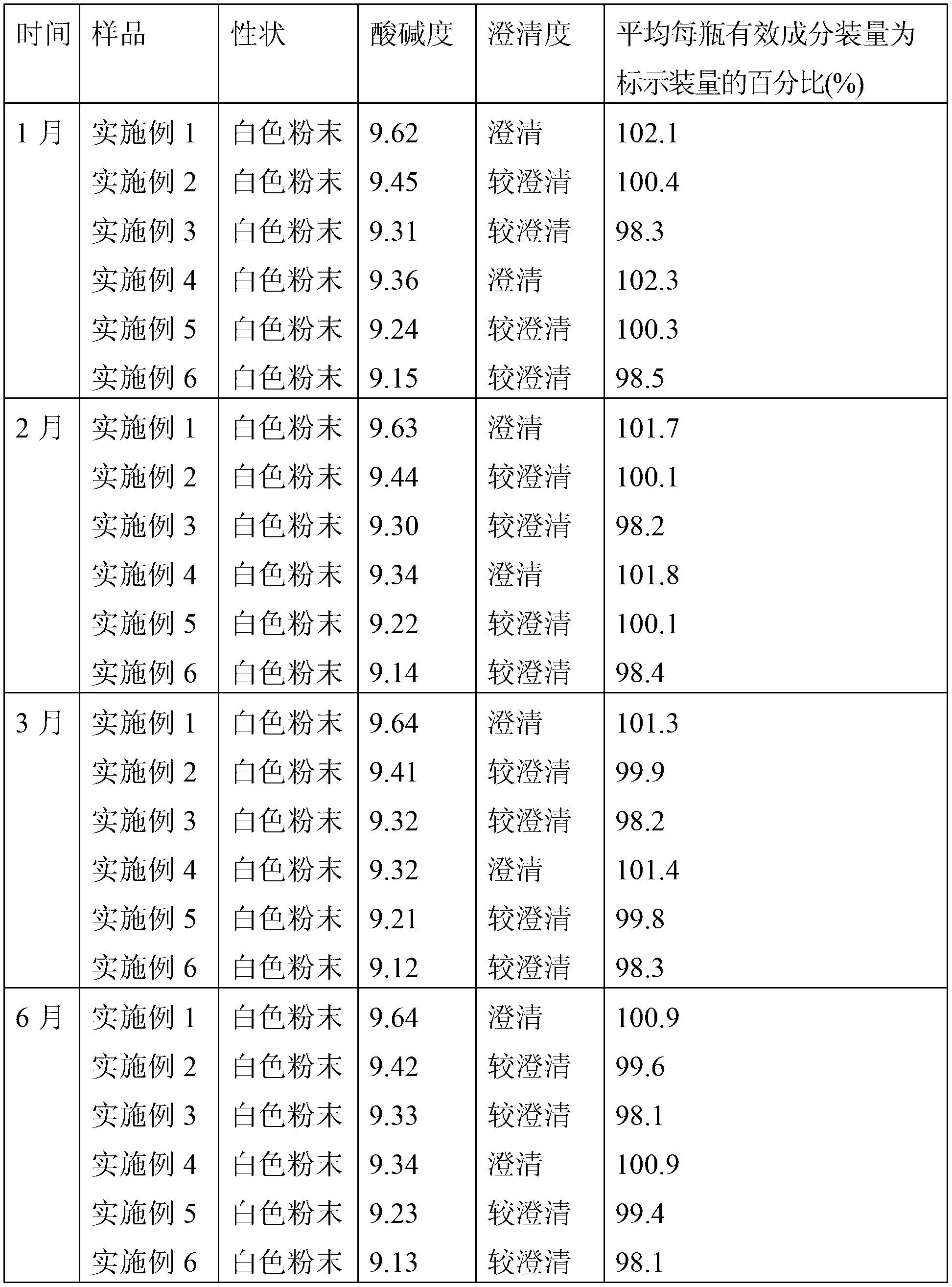
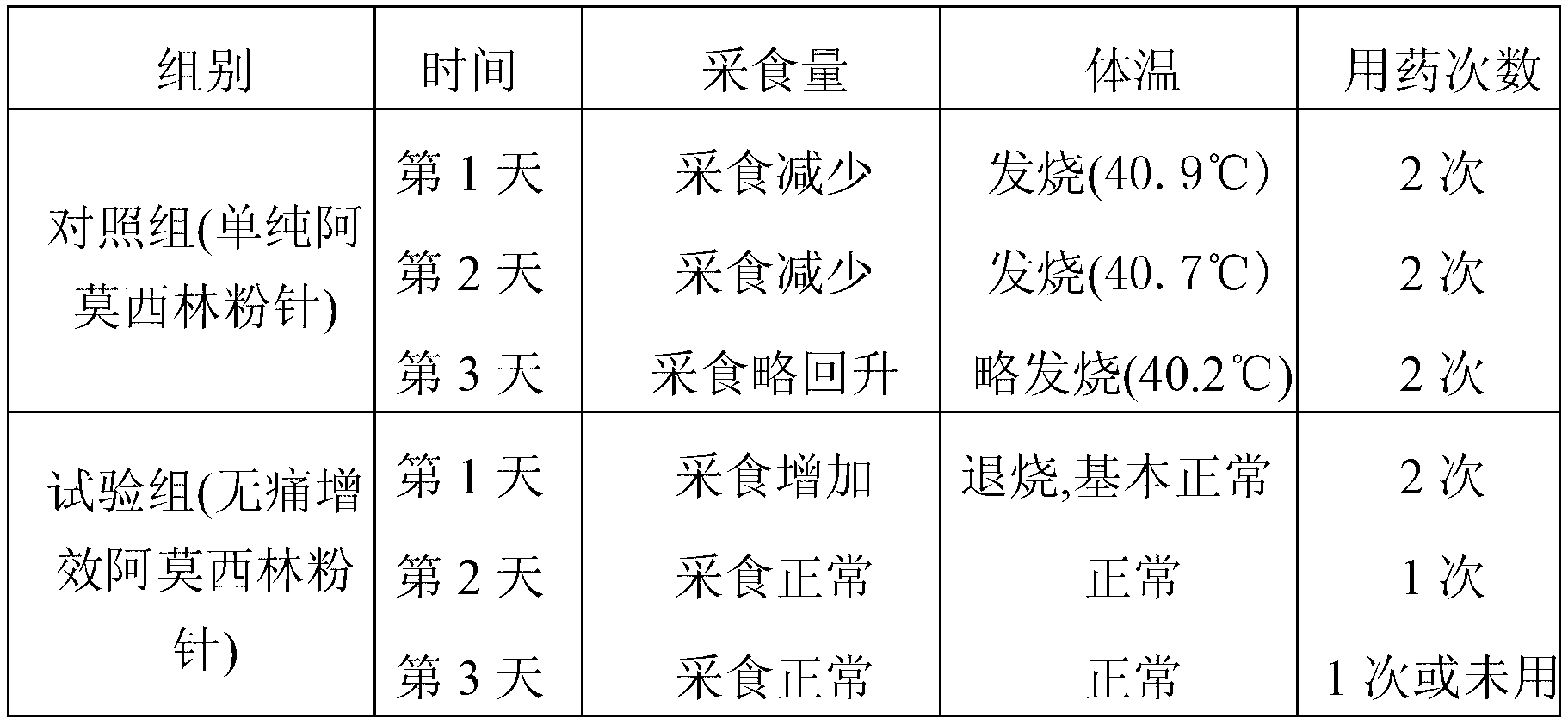
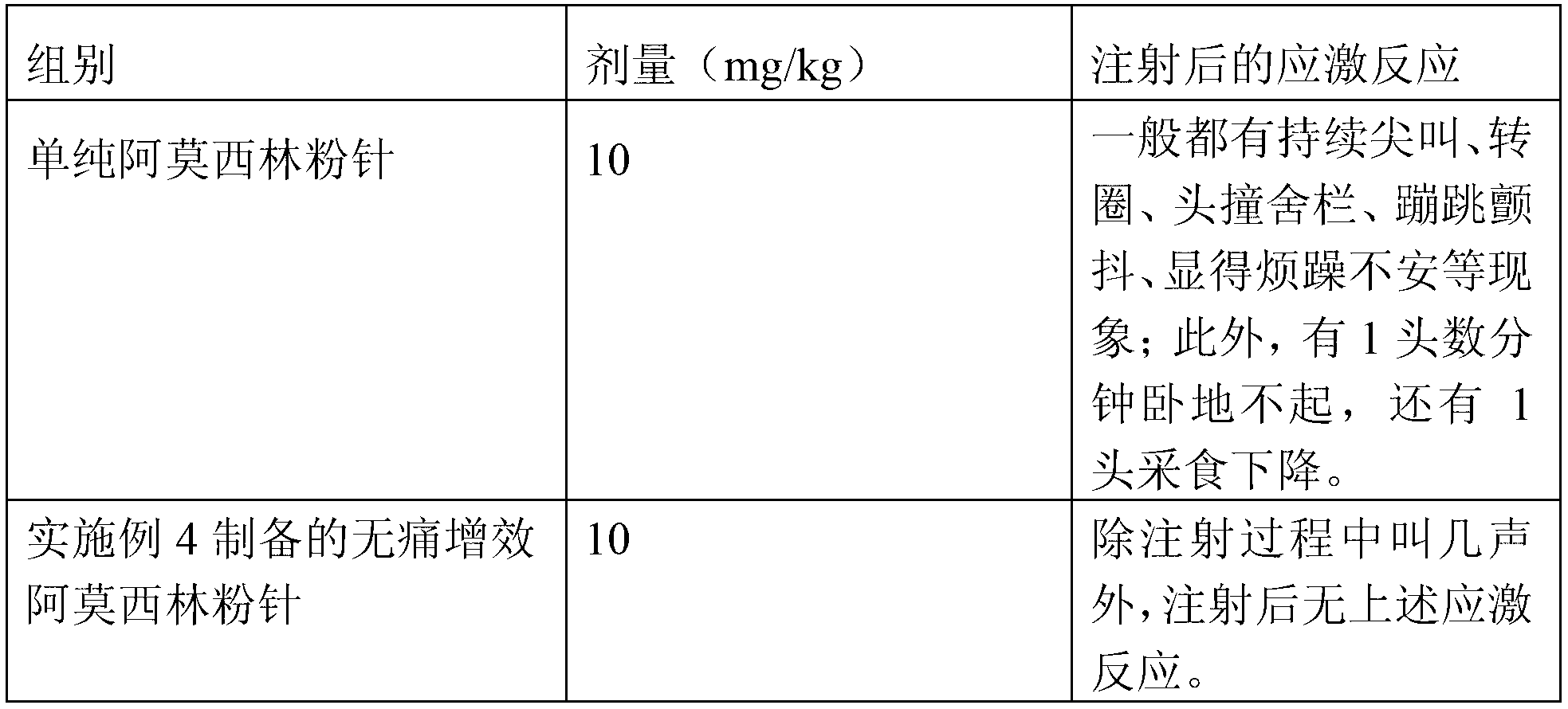
Painless synergistic amoxicillin powder injection for animals and preparation method thereof
InactiveCN103169668ADefinite curative effectThere is a high demand for clinical applicationsAntibacterial agentsPowder deliveryAmoxicillin SodiumSolvent
The invention belongs to the field of the medicines for animals and discloses a painless synergistic amoxicillin powder injection for animals and a preparation method thereof. The powder injection comprises the following components in parts by weight: 48-52 parts of amoxicillin or amoxicillin sodium (active ingredient), 10-30 parts of a cosolvent, 1-3 parts of an analgetic, 5-8 parts of probenecid sodium and 1-2 parts of an antifebrile anti-inflammatory analgesic. The preparation method of the powder injection comprises the following steps of: in parts by weight, evenly mixing 1-3 parts of analgetic, 5-8 parts of probenecid sodium and 1-2 parts of antifebrile anti-inflammatory analgesic together, and then evenly mixing the mixture with 48-52 parts of amoxicillin or amoxicillin sodium (active ingredient) and 10-30 parts of cosolvent, thereby obtaining the painless synergistic amoxicillin powder injection. The amoxicillin powder injection prepared by the preparation method provided by the invention solves the problems of strong injection pricking stress, poor effects of anti-inflammation and analgesia, and heat elimination and defervescence, ingestion reduction after the administration of the drug, and the like; and the product is highly required in clinical application, and is wide in market prospect and huge in social benefit.
Owner:广东省天宝生物制药有限公司
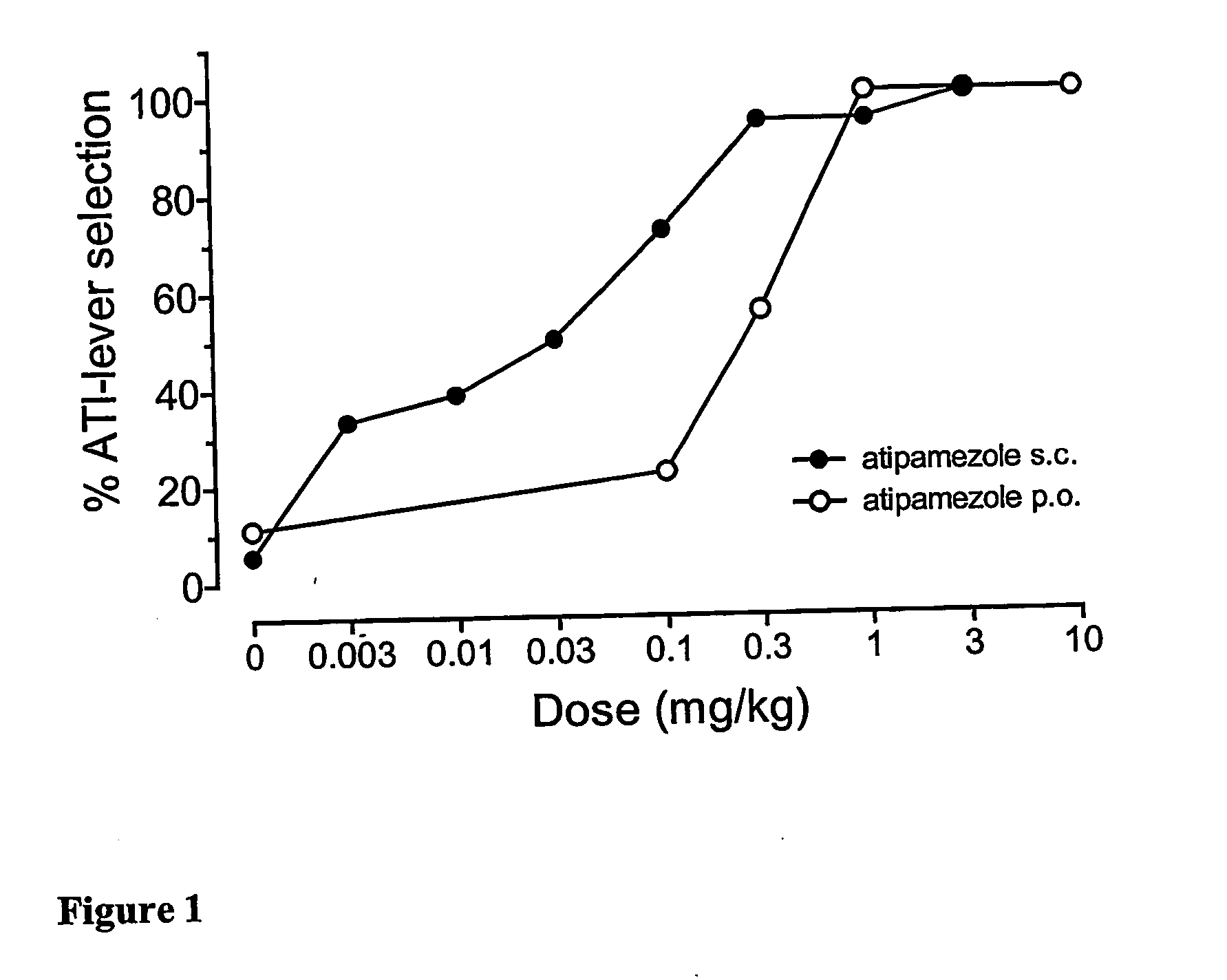
Treatment of dependence and dependence related withdrawal symptoms
A method for treatment of dependence and dependence related withdrawal symptoms caused by the discontinuation of subacute or chronic use of psychostimulant agents, to ease a patient's withdrawal from the psychostimulants with an alpha2-adrenoceptor antagonist or a pharmaceutically acceptable ester or salt thereof.
Owner:ORION CORPORATION
Beta2-receptorexcitant and preparation method and application thereof
ActiveCN106279018AOrganic active ingredientsOrganic chemistryFree formObstructive Pulmonary Diseases
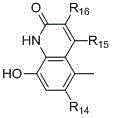
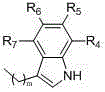
The invention relates to a following formula I showed compound in a free form, a salt form or a solvated form and having a beta2-receptor exciting effect. II and III are contained in R1, Ar are following formula groups IV and V, definition of R1, R2, R3, R4, R5, R6, R7, R8, R9, R10, R13, R14, R15, R16, m and o is shown in the description. The invention further relates to a preparation method of the compound and application of a compound serving as a drug, in particular to treatment of diseases such as a chronic obstructive pulmonary disease, asthma and heart failure the aspects of a respiratory and cardiovascular system and a cardiovascular system.
Owner:SHENYANG PHARMA UNIVERSITY
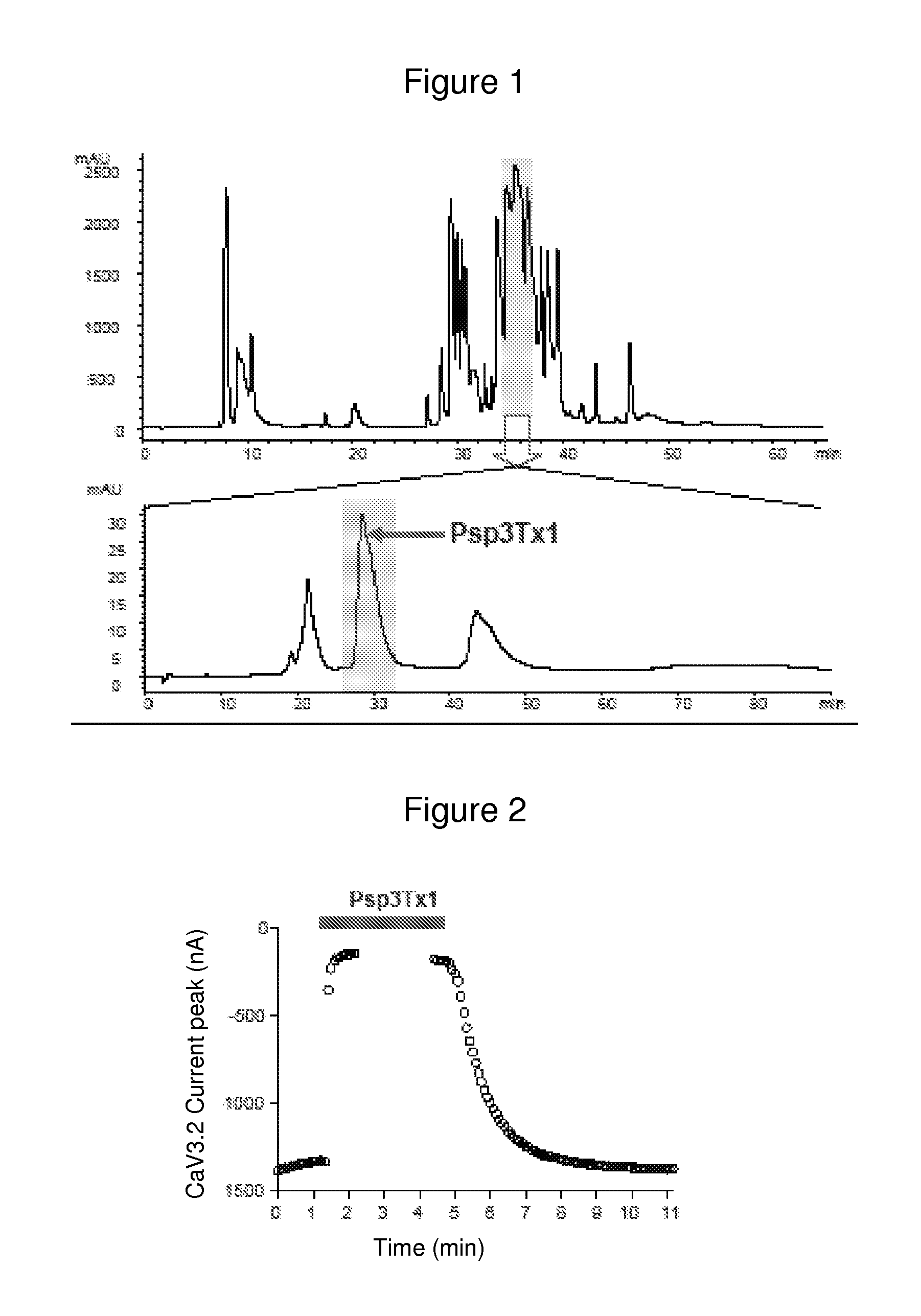
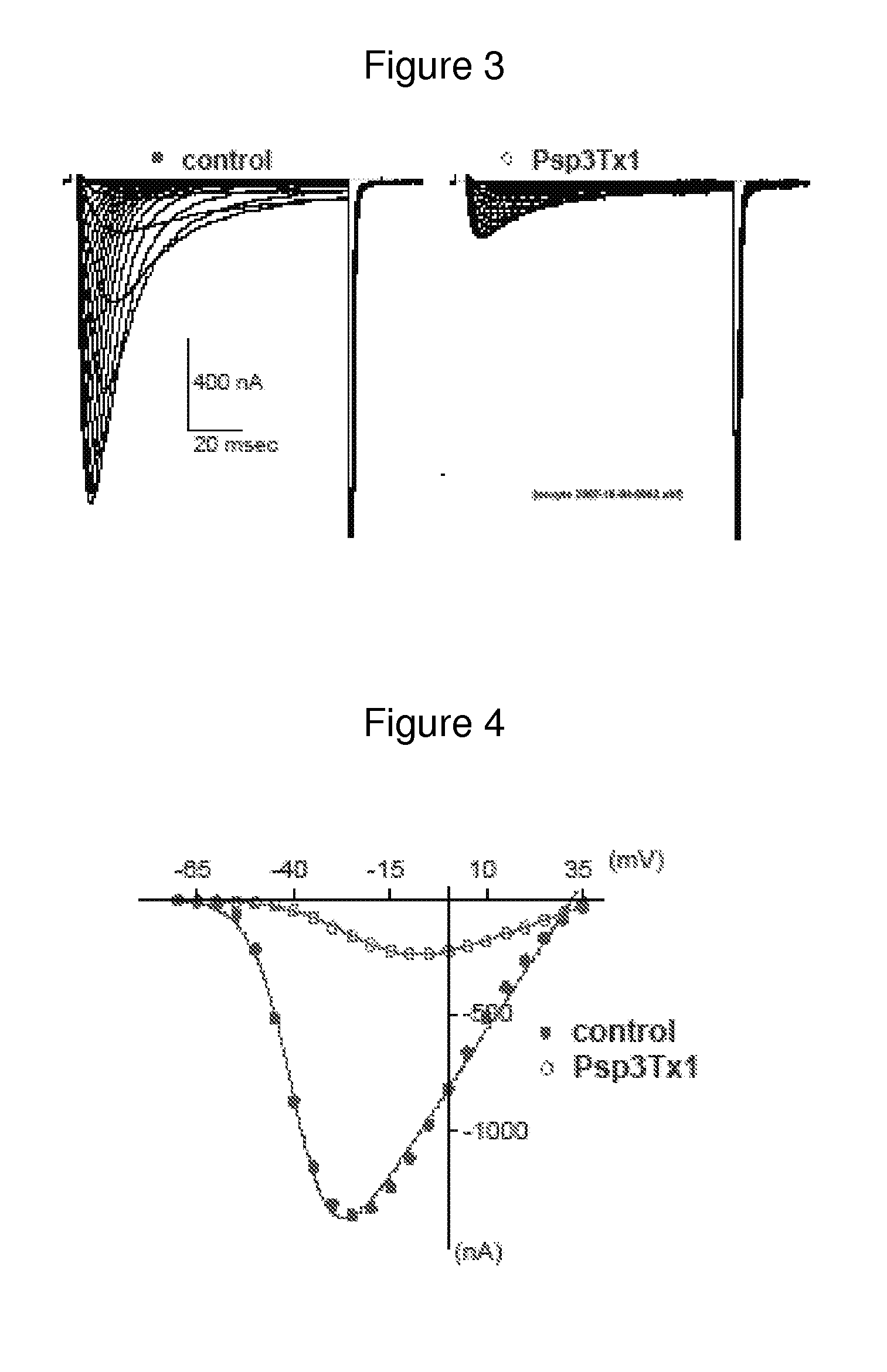
Identification of novel antagonist toxins of t-type calcium channel for analgesic purposes
InactiveUS20120040909A1Lower blood pressureInhibit cell proliferationSenses disorderNervous disorderT-type calcium channelAgonist
A peptide with the following sequence YCQKFLWTCDSERPCCEGLVCRLWCKIN (SEQ ID NO 1) or a derivative thereof, and nucleic acids coding for the peptide having the sequence (SEQ ID NO 1). Also the use of this peptide as an antagonist and / or reverse agonist of T-type calcium channels. A use of the peptide for preparing a drug, in particular an analgesic one.
Owner:CENT NAT DE LA RECHERCHE SCI +1
Apparatus, method and drug products for providing a conscious patient relief from pain and anxiety associated with medical or surgical procedures
Disclosed is drug delivery for facilitating medical and / or procedures that are performed without “general anesthesia,” which is also described in the specification as the state of patient “unconsciousness” resulting from a drug administered by an anesthetist or anesthesiologist. Mixtures of sedative and analgesic drugs are adapted for safe and effective administration by devices to provide and maintain drug infusions that do not push the patient into unconsciousness and / or general anesthesia. Drug delivery devices are also disclosed that include the use of stored parameters and / or values that correlate to drug mixture delivery during a procedure, and a patient health monitor to measure and send signals regarding a patient health condition to a processor.
Owner:SCOTT LAB
Method of producing a novel opioid peptide
InactiveUS20120004180A1Good analgesic effectConserve and increases their analgesic activityNervous disorderTetrapeptide ingredientsPharmacophoreRheumatism
The use of opioid peptides of a novel structure is claimed which, in addition to a pharmacophore, additionally contain structural elements reactive with tachykinin receptors. Due to the synergistic reactivity of the opioid with an additional element, an increased analgesic activity is obtained facilitating protracted effective use due to decreased drug tolerance effects. The drugs may particularly be of use in the treatment of chronic pain as effective analgesics during inflammation caused by rheumatism, gout, neurodegenerative states, post-surgical and post-traumatic inflammations or ones induced by tumours.
Owner:LIPKOWSKI ANDREJ
Pre-operative carbohydrate-rich beverage composition and methods of treatment
ActiveUS11102994B2Add flavorProlong lifeDispersion deliveryHydroxy compound active ingredientsNervous systemVasoconstrictor Agents
Provided herein are beverage compositions to be ingested by a patient prior to administration of anesthesia or sedation comprising: a) one or more carbohydrates, wherein the total Calories available from the carbohydrates is at least about 200 and wherein the one or more carbohydrates are the sole source of significant Calories in the beverage; b) an acid, in a quantity sufficient to result in a pH of at least about 3.7 to about 4.5 and to enhance the shelf life and flavor of the beverage; c) a central nervous system (CNS) stimulant that is also a cerebro-vasoconstrictor; d) a sweetener and e) water, wherein the beverage composition, when ingested at least about two hours prior to administration of anesthesia or sedation is effective to relieve one or more symptoms associated with prolonged fasting. Further provided herein are methods of using the pre-operative beverage compositions disclosed herein in preparation for the safe induction of anesthesia or sedation in a patient before surgery.
Owner:CLEARFAST INC
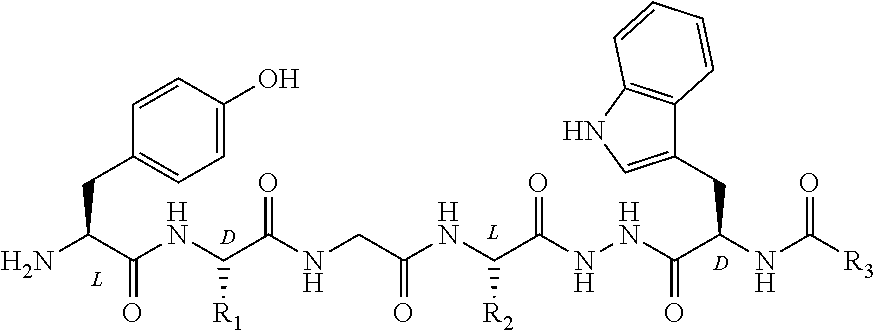
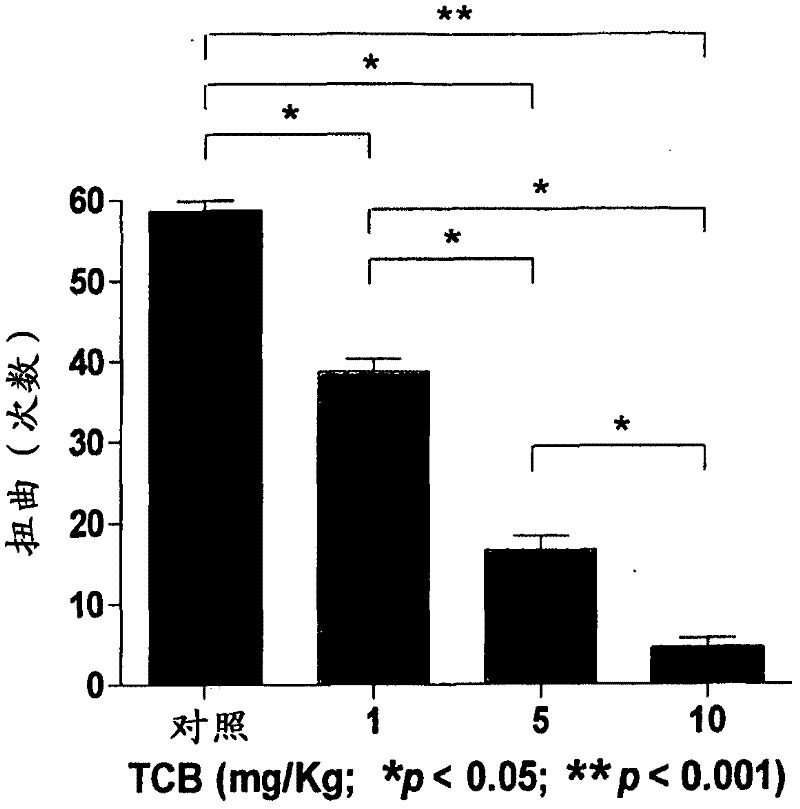
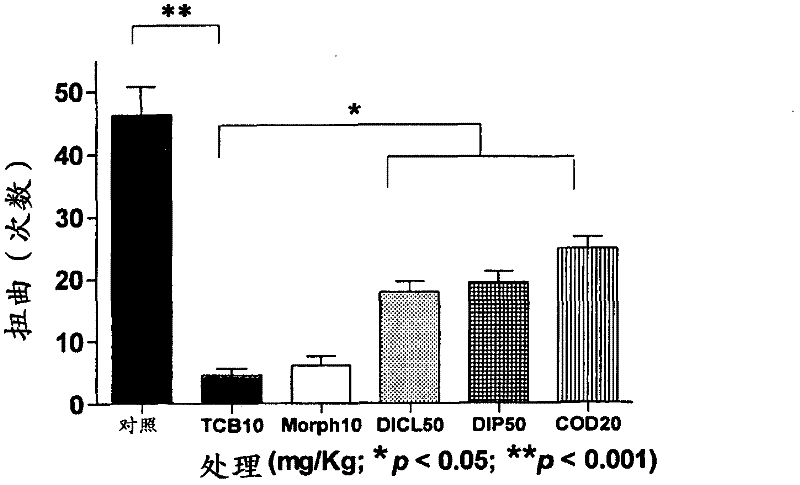
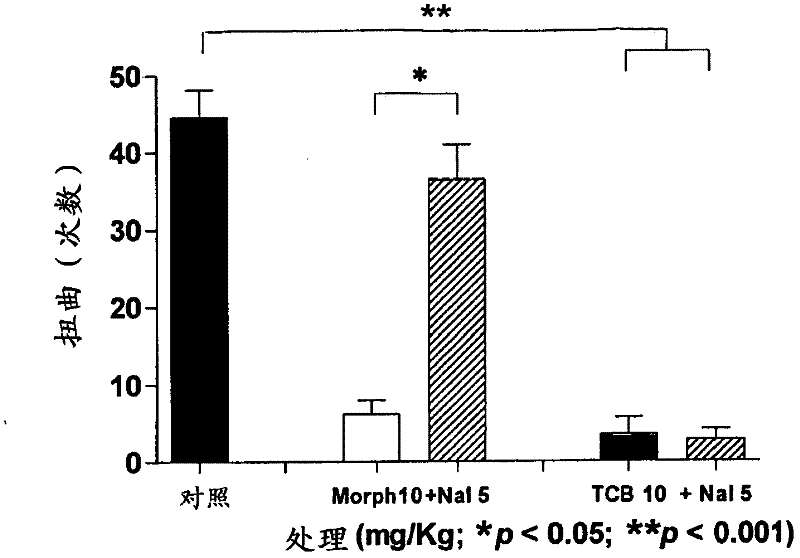
Use of telocinobufagin as an analgesic in the treatment of acute and chronic pains
The present invention is directed to the use of telocinobufagin, or its pharmaceutically acceptable derivatives, in the manufacture of a medicament for the treatment or prevention of acute and chronic pains. The present invention also refers to a pharmaceutical composition comprising an effective amount of telocinobufagin; also provides a method to induce analgesia in response to acute and chronic pains that comprehends administering an effective amount of telocinobufagin to human beings or animals. According to the outcomes of in vivo assays, telocinobufagin is more potent than morphine, though without presenting the known side effects of opioids. In addition, in vivo and in vitro essays showed that TBC does not present cardiotoxicity.
Owner:CRISTALIA PROD QUI FARM LTDA +1
Methods of treating pain
The invention relates to methods for treating pain disorders including neuropathic and inflammatory pain and to methods to reduce or eliminate nociceptive tolerance induced by opiate analgesic use by administering an agent that suppresses or blocks S1P biological activity.
Owner:SAINT LOUIS UNIVERSITY
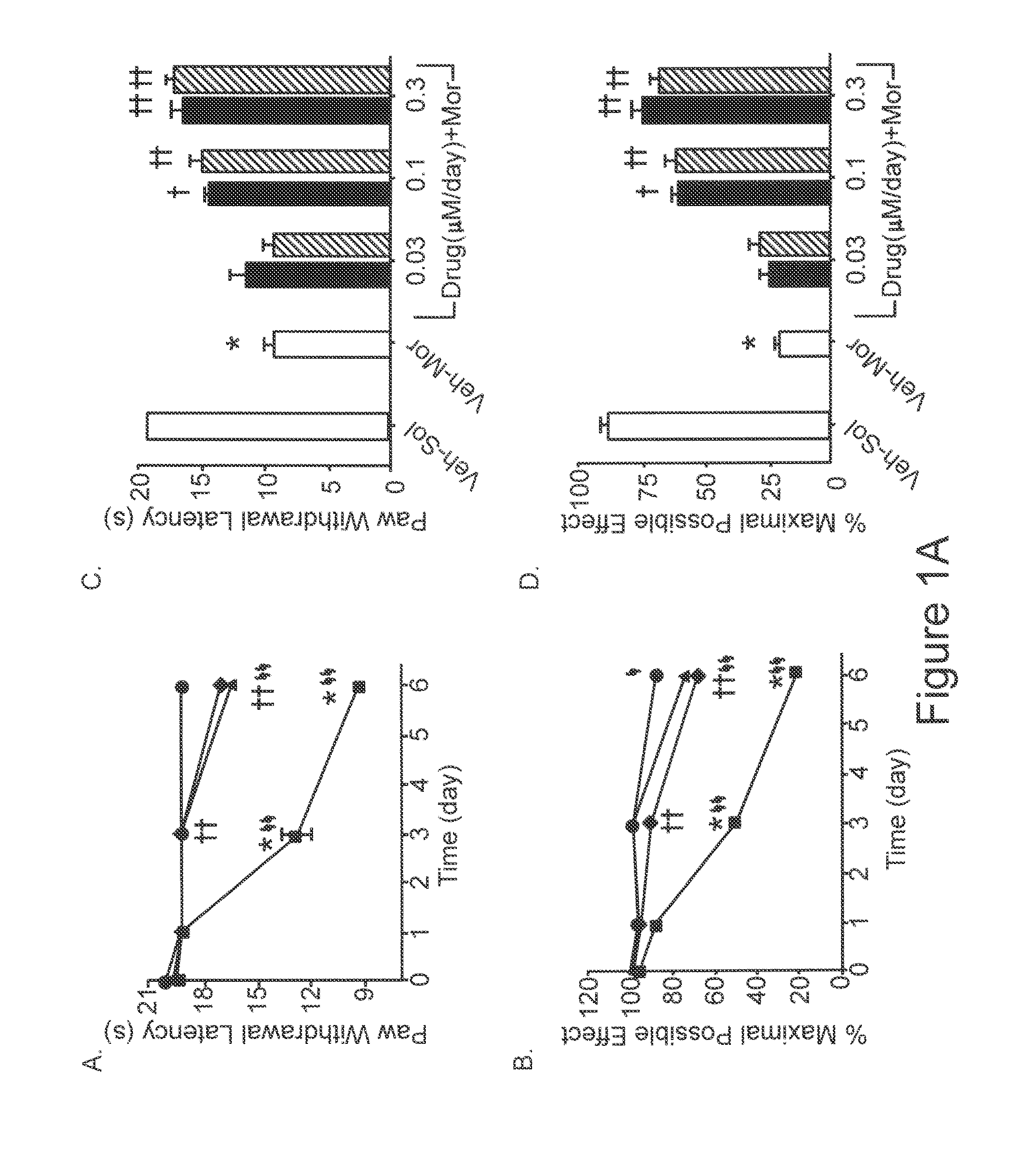
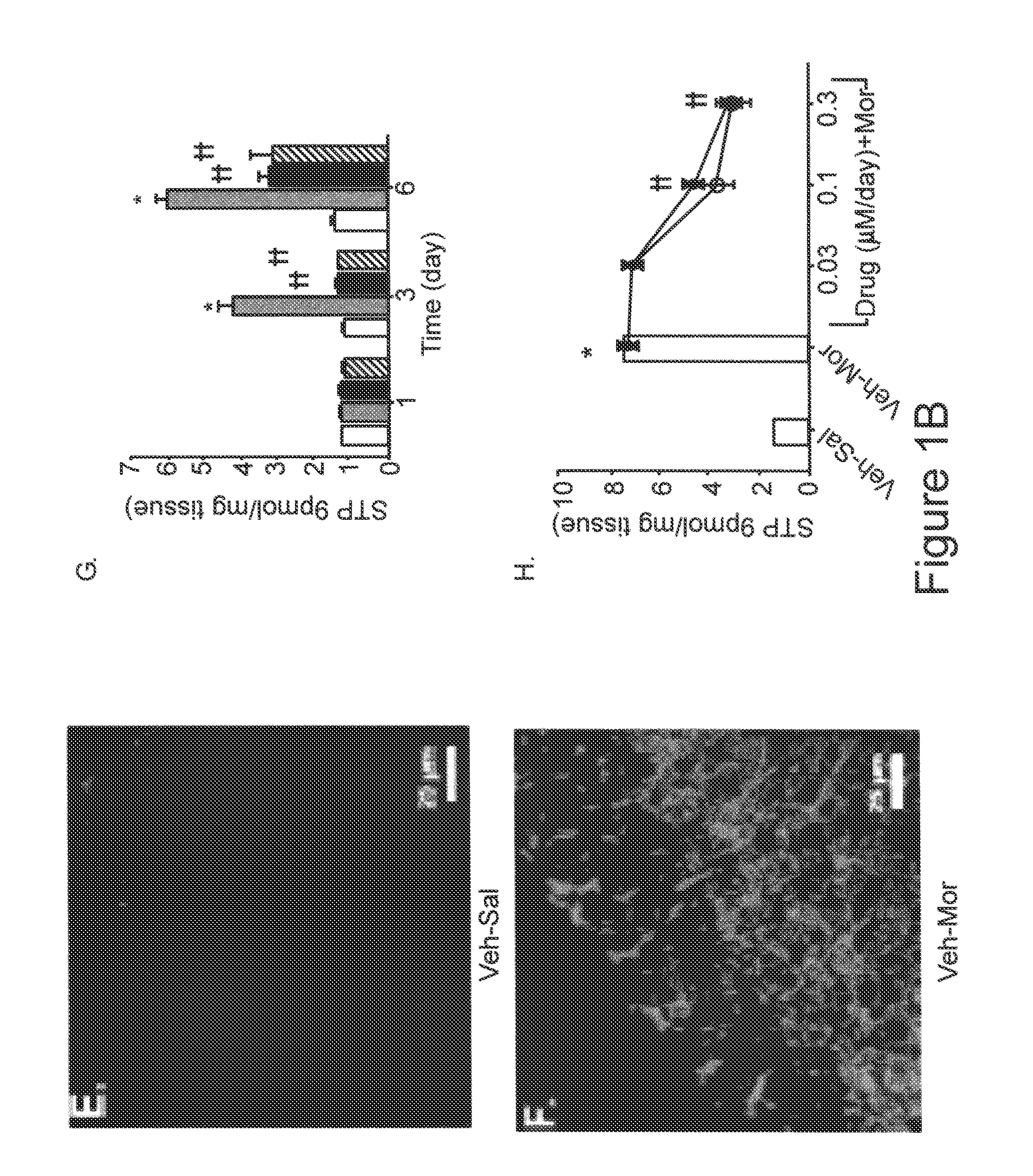
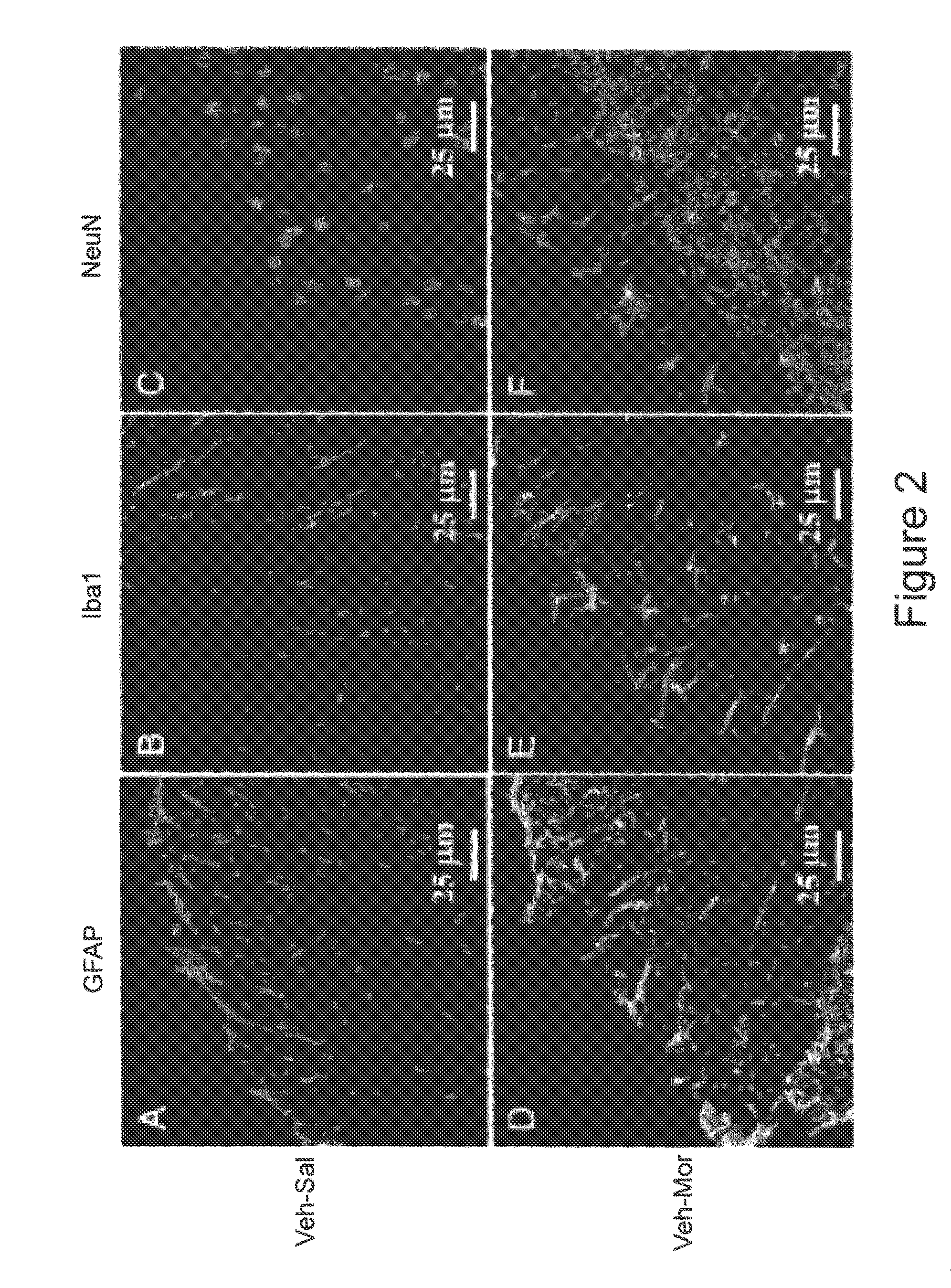
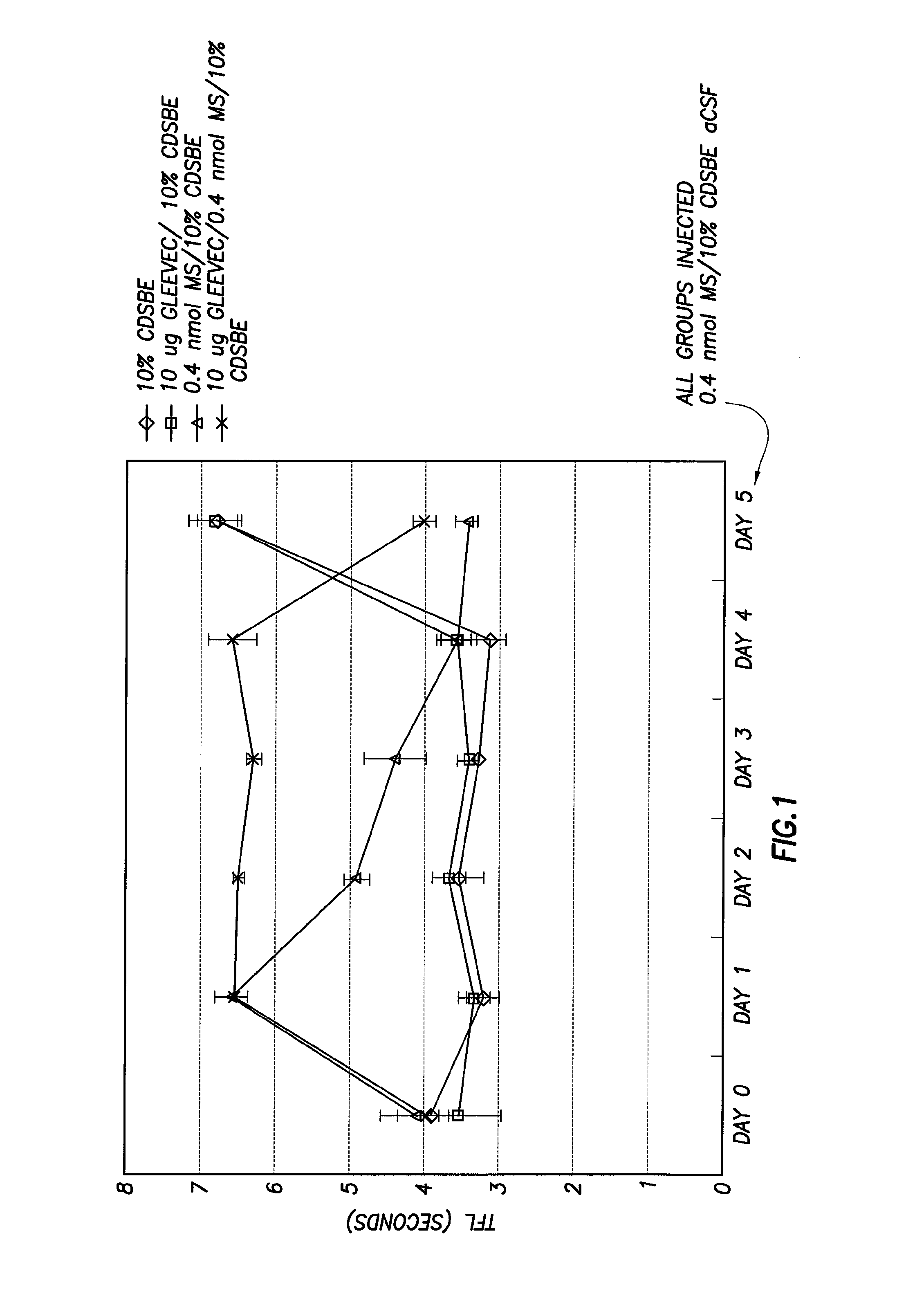
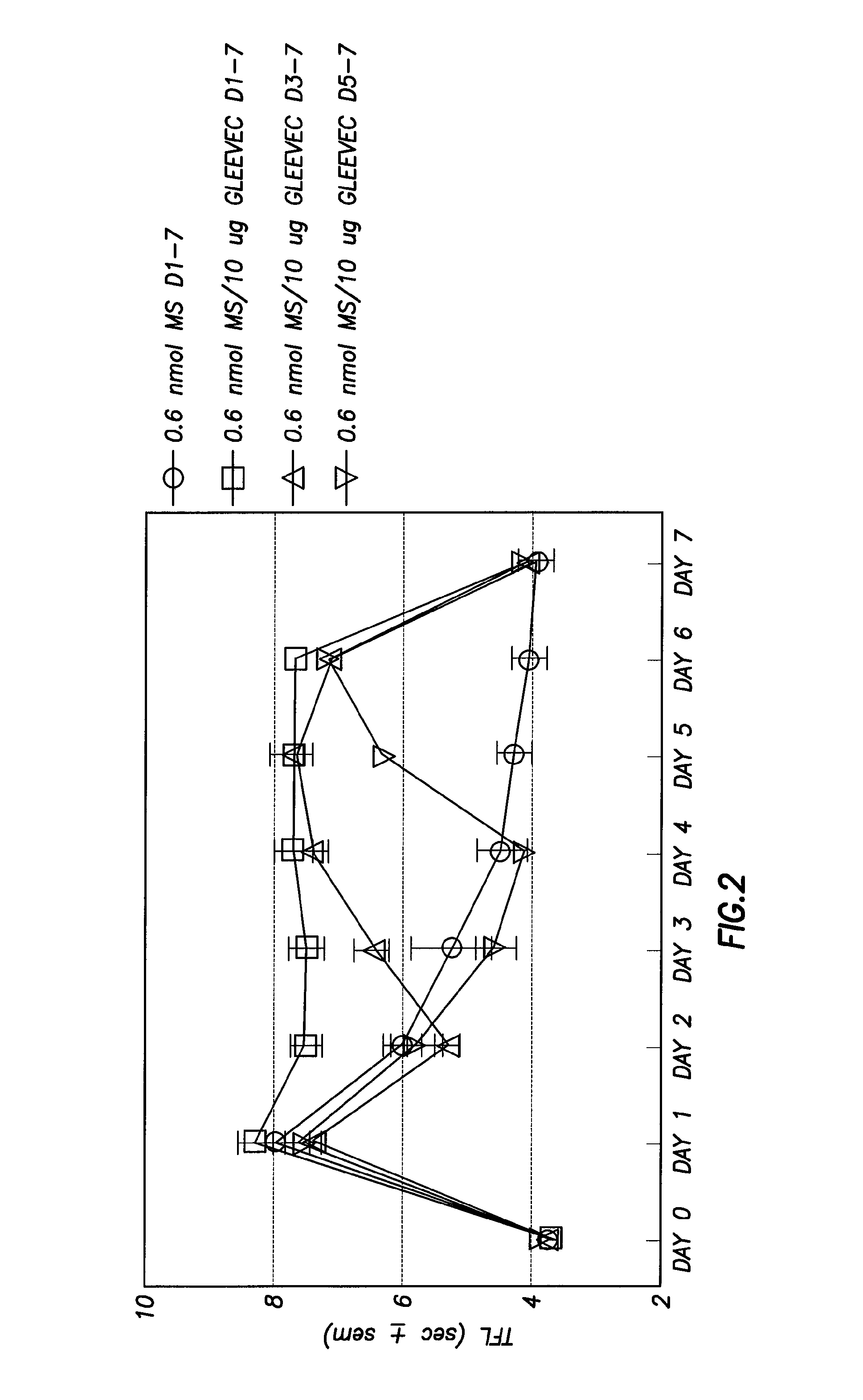
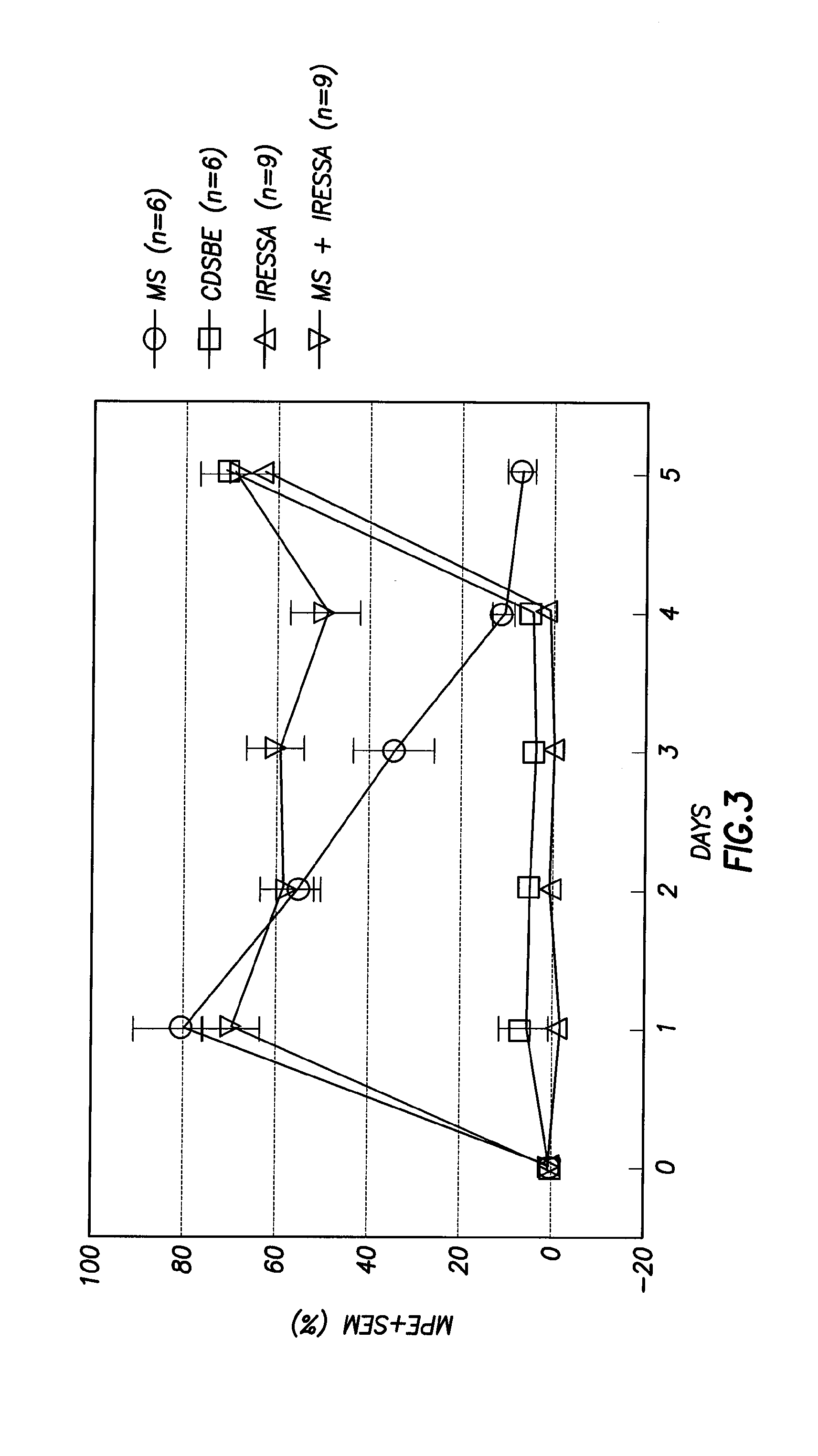
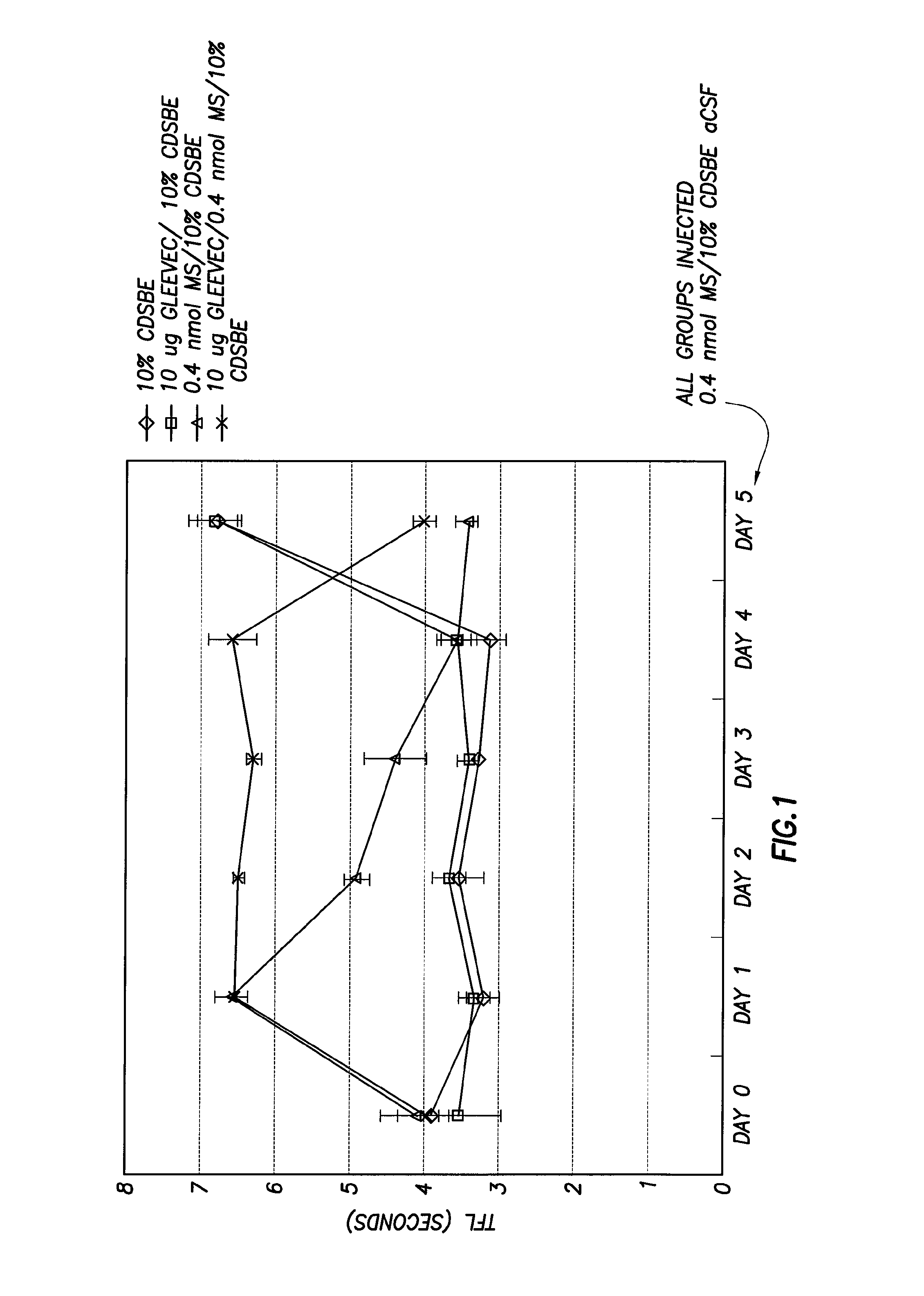
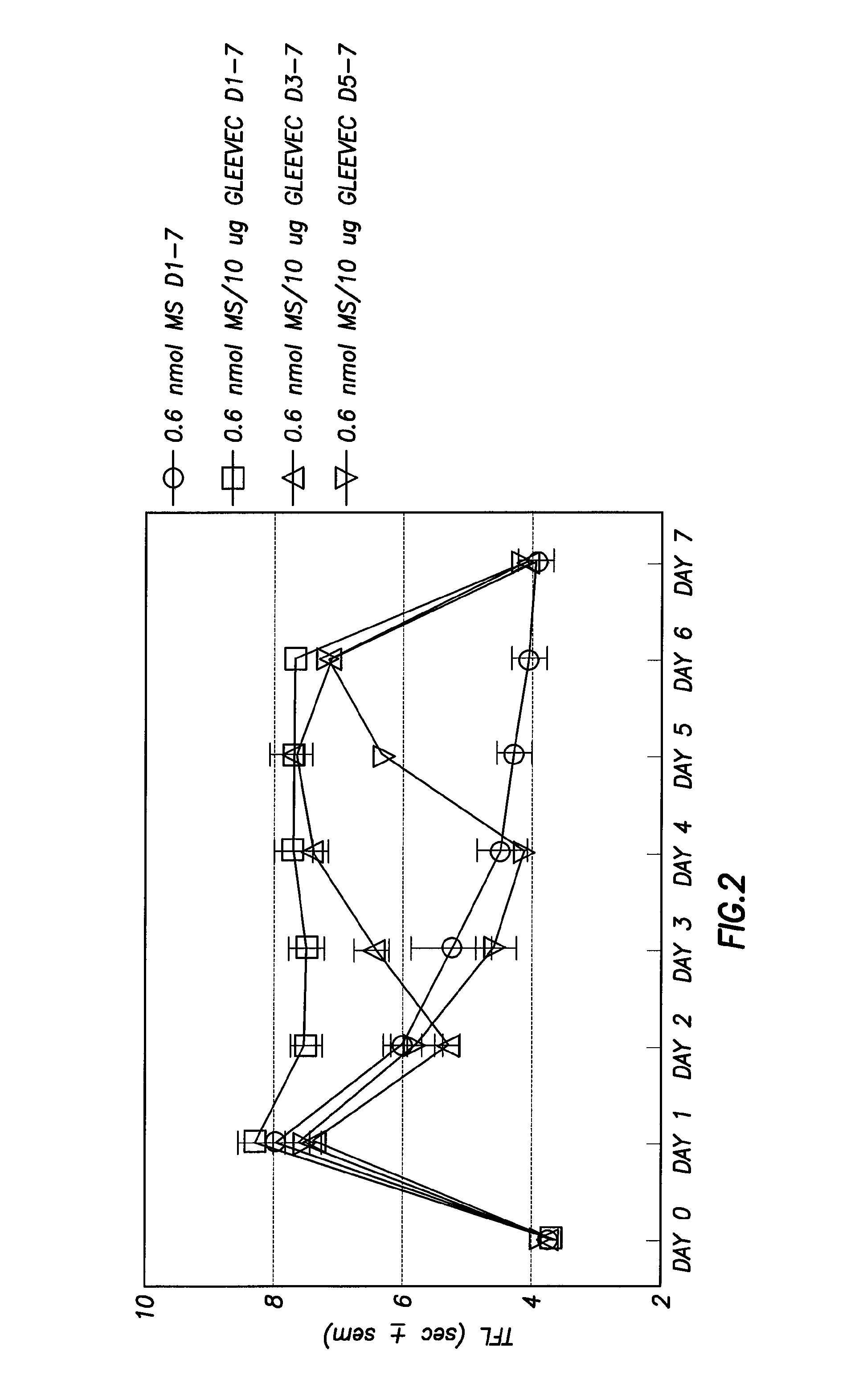
Methods of Treatment of Opioid Tolerance, Physical Dependence, Pain and Addiction With Inhibitors of Certain Growth Factor Receptors
InactiveUS20100210709A1Preventing and reducing and reversing opioid toleranceCombination therapy can be facilitatedBiocideOrganic active ingredientsPhysical dependenceMedicine
Methods of preventing the development and reversing or partially reversing opioid tolerance in a subject are provided herein. Such methods include the step of administering to a subject in need thereof a therapeutically effective amount of a PDGFR modulator or EGFR modulator alone or together with an opiate analgesic. The methods can also be used for the treatment of refractory neuropathic pain, physical dependence or addiction.
Owner:BOARD OF RGT THE UNIV OF TEXAS SYST
Methods of treatment of opioid tolerance, physical dependence, pain and addiction with inhibitors of certain growth factor receptors
InactiveUS8501740B2Preventing, reducing and/or reversing opioid toleranceCombination therapy can be facilitatedOrganic active ingredientsBiocidePhysical dependenceMedicine
Methods of preventing the development and reversing or partially reversing opioid tolerance in a subject are provided herein. Such methods include the step of administering to a subject in need thereof a therapeutically effective amount of a PDGFR modulator or EGFR modulator alone or together with an opiate analgesic. The methods can also be used for the treatment of refractory neuropathic pain, physical dependence or addiction.
Owner:BOARD OF RGT THE UNIV OF TEXAS SYST
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com