Patents
Literature
90 results about "Capsazepine" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
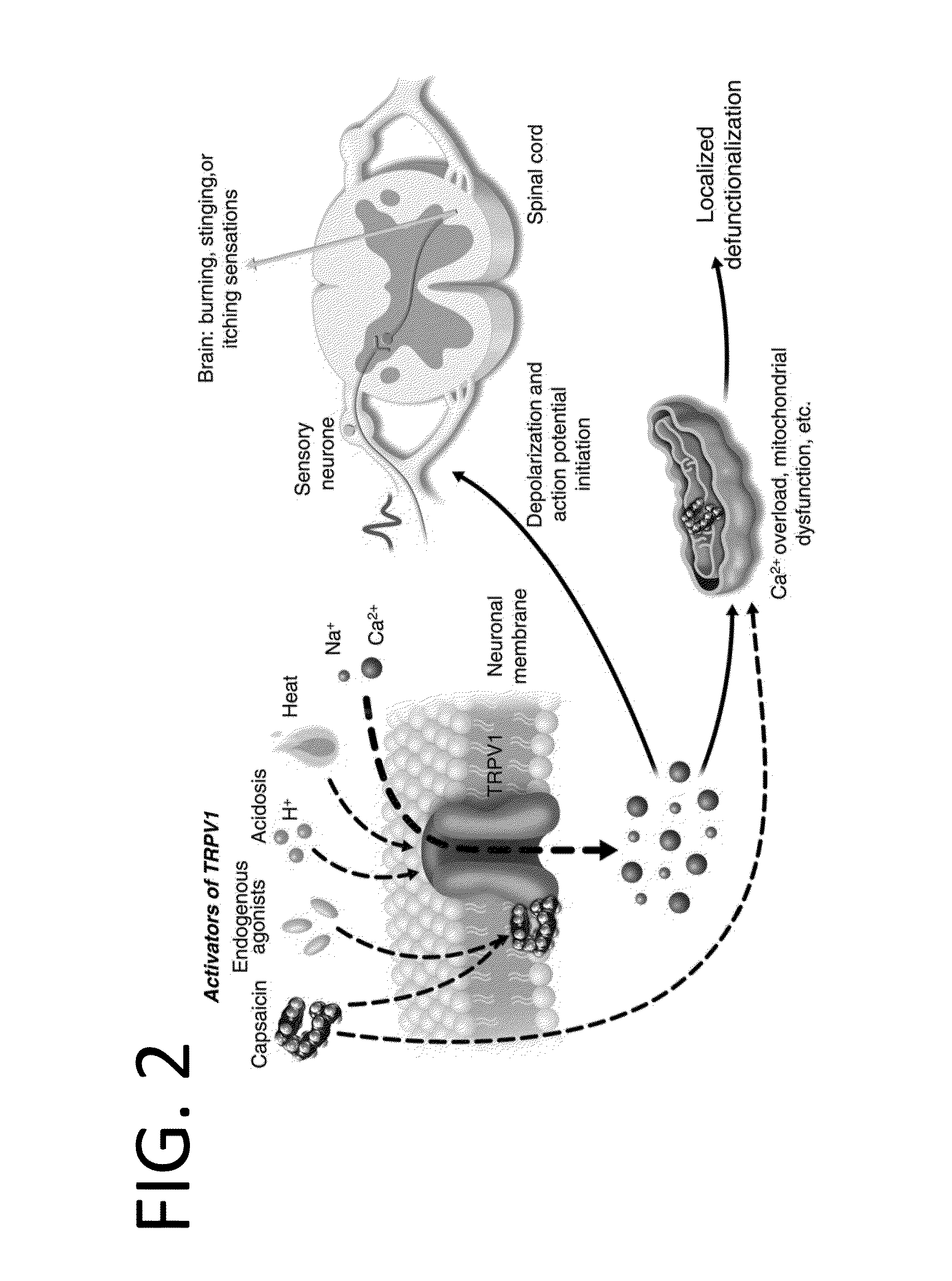
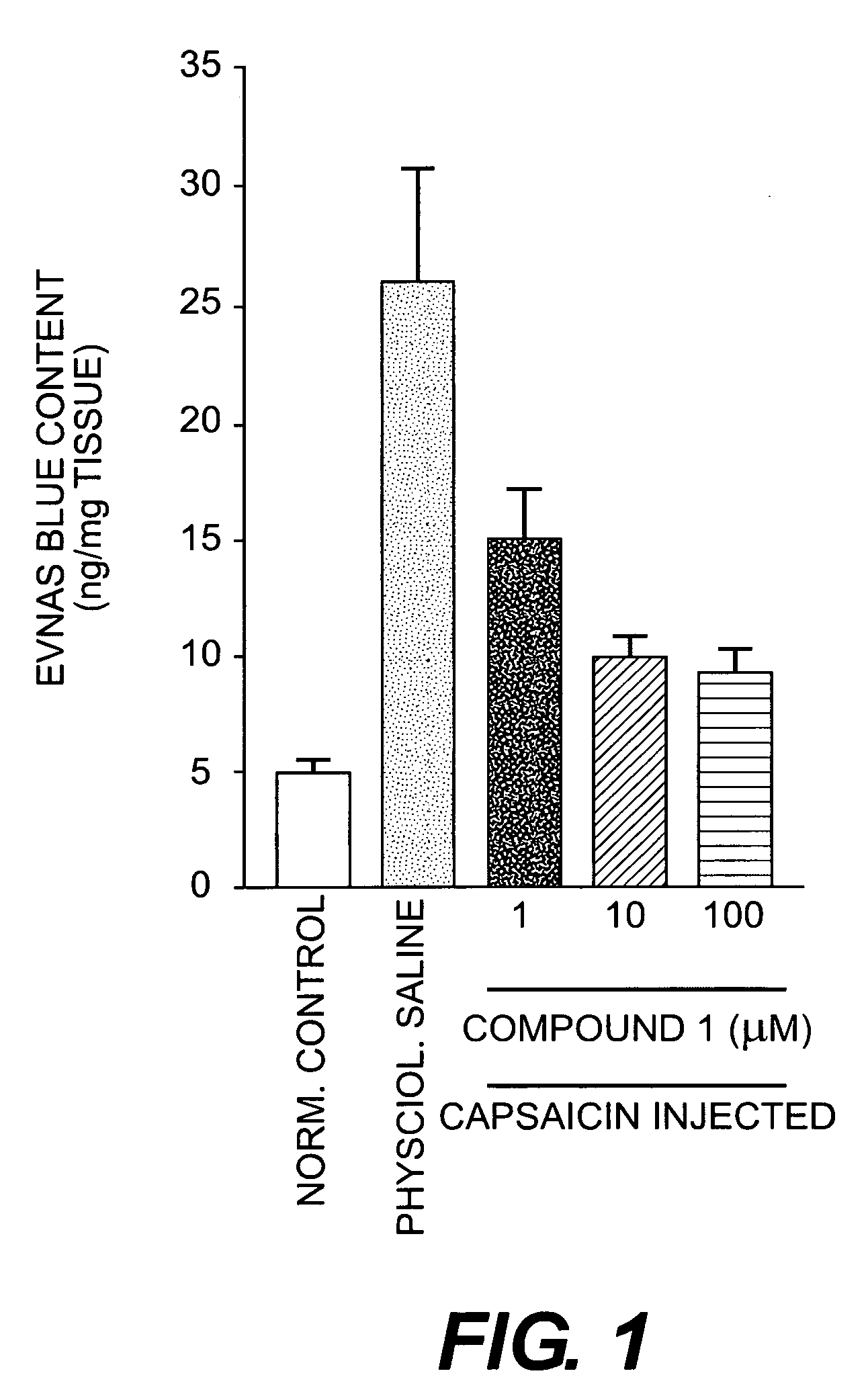
Capsazepine is a synthetic antagonist of capsaicin. It is used as a biochemical tool in the study of TRPV ion channels.
Esters of capsaicin for treating pain
ActiveUS20080020996A1Reduce generationImprove lipophilicityAntibacterial agentsBiocideSolubilityIrritation
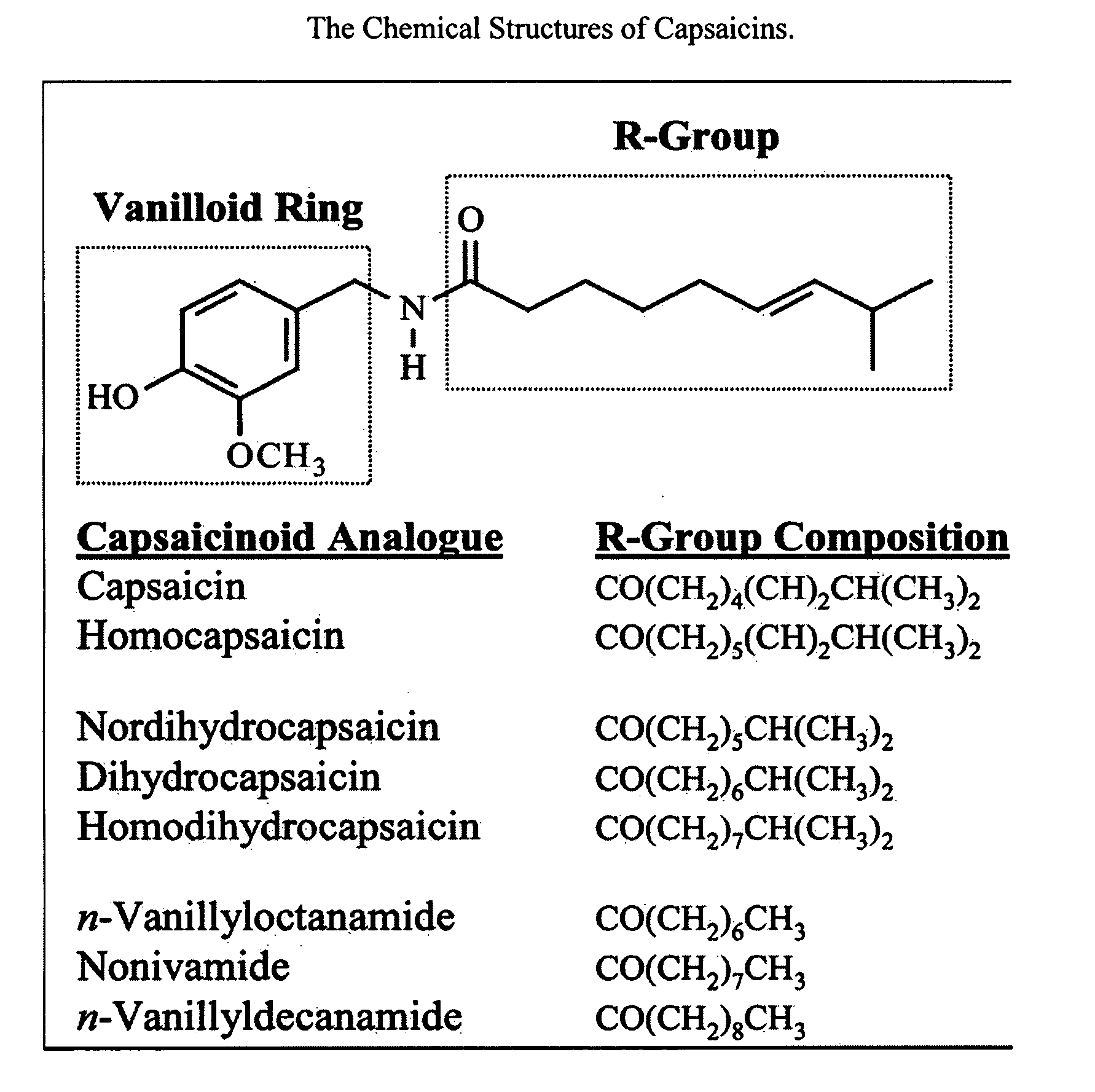
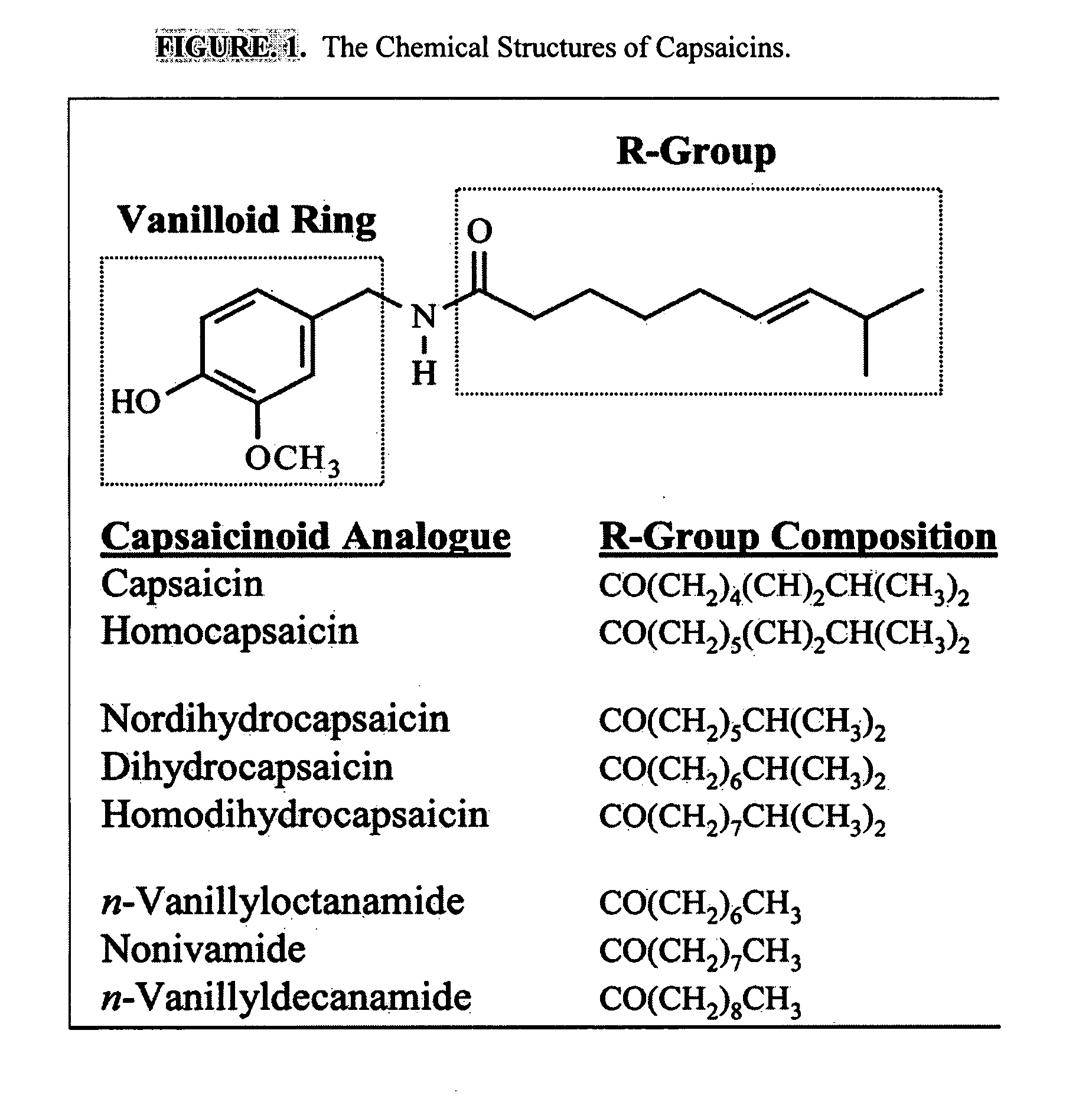
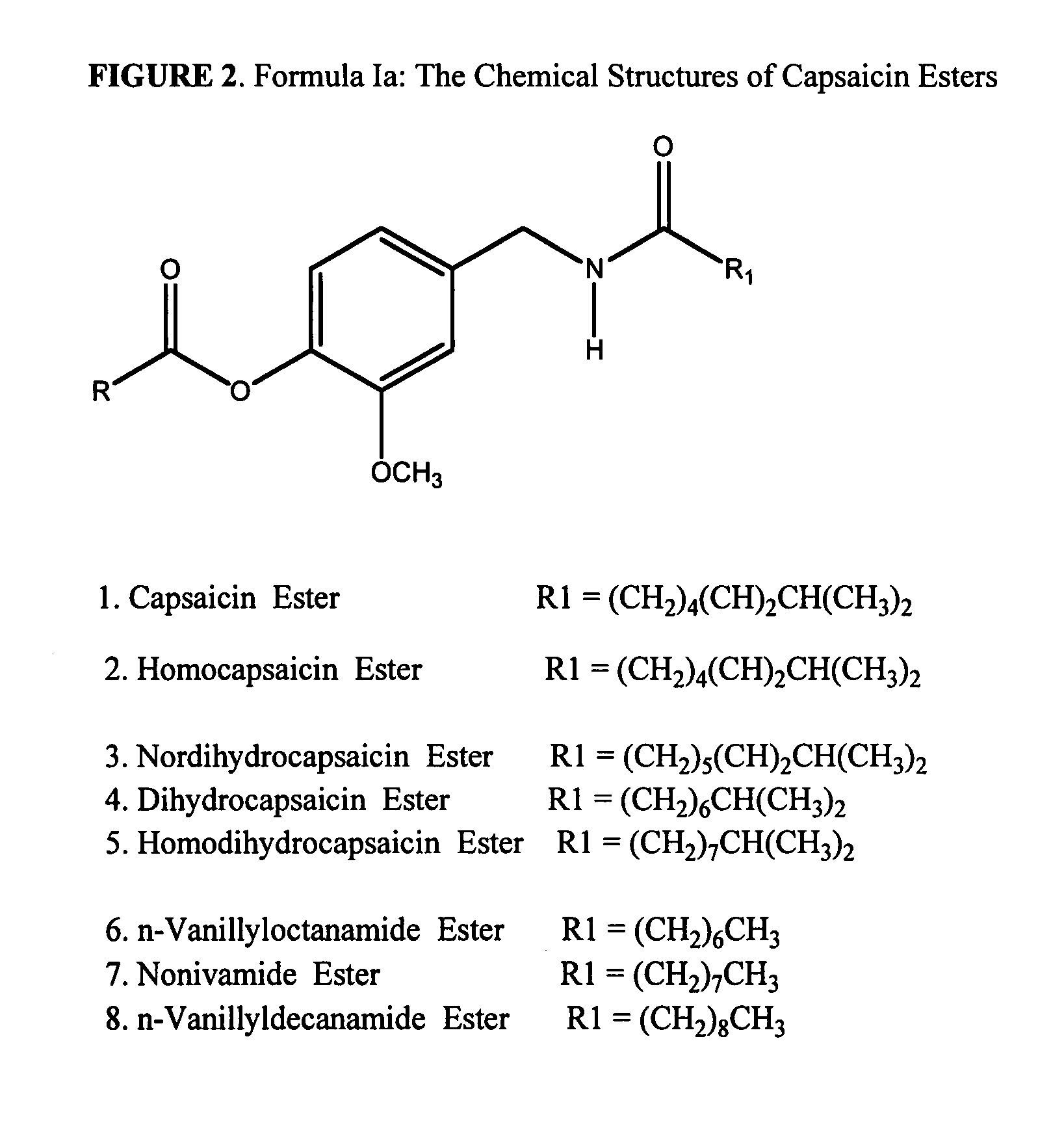
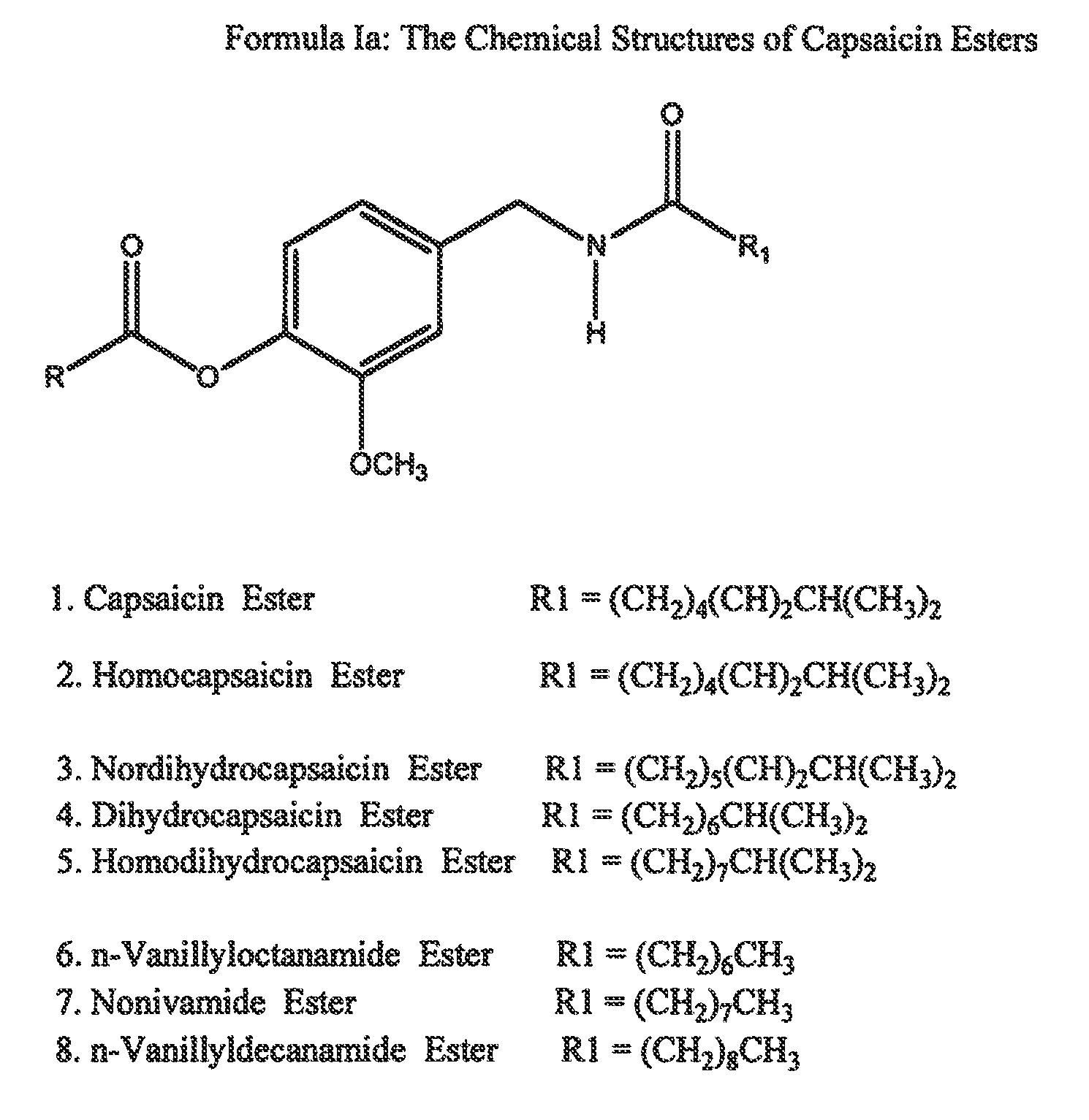
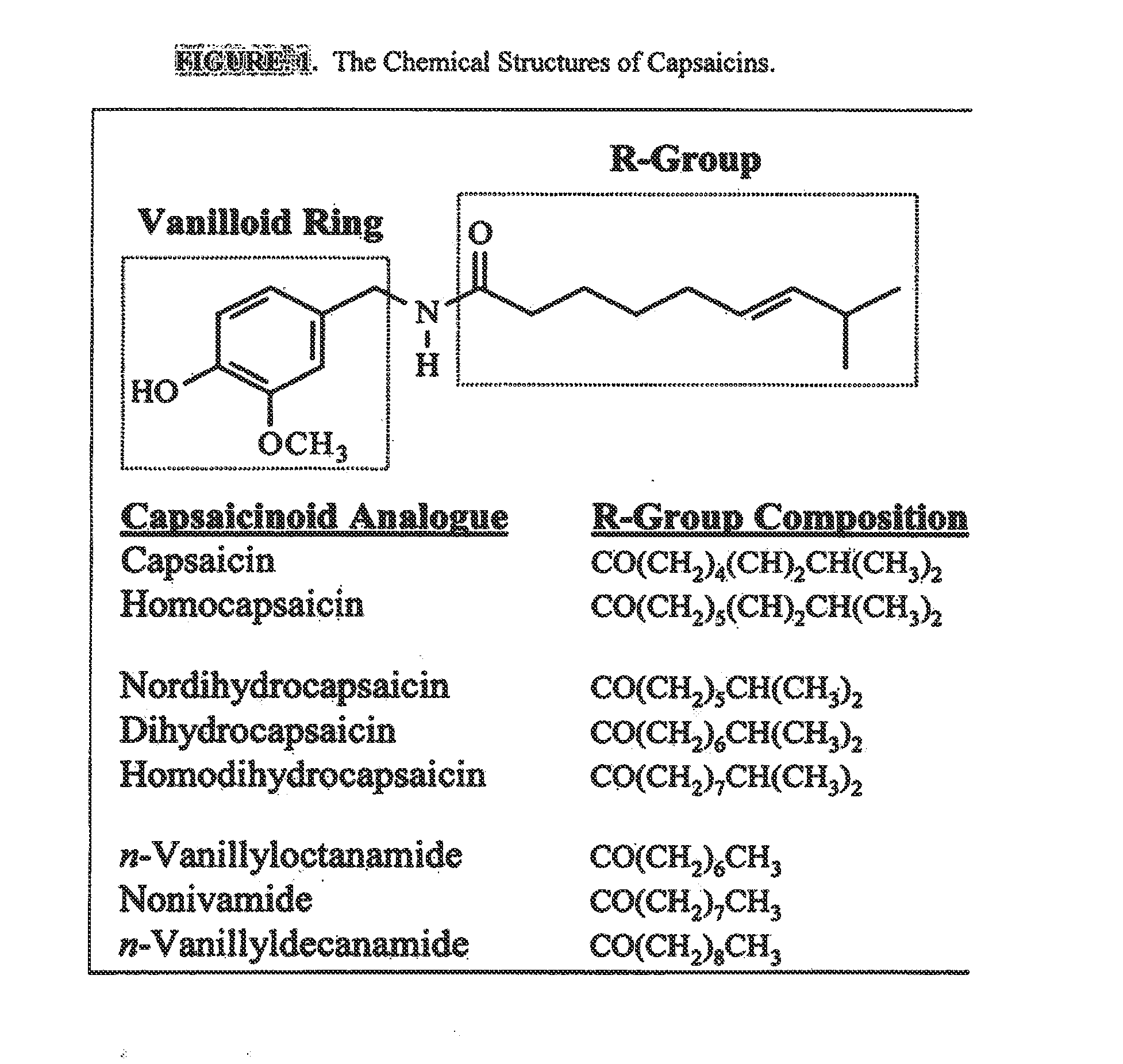
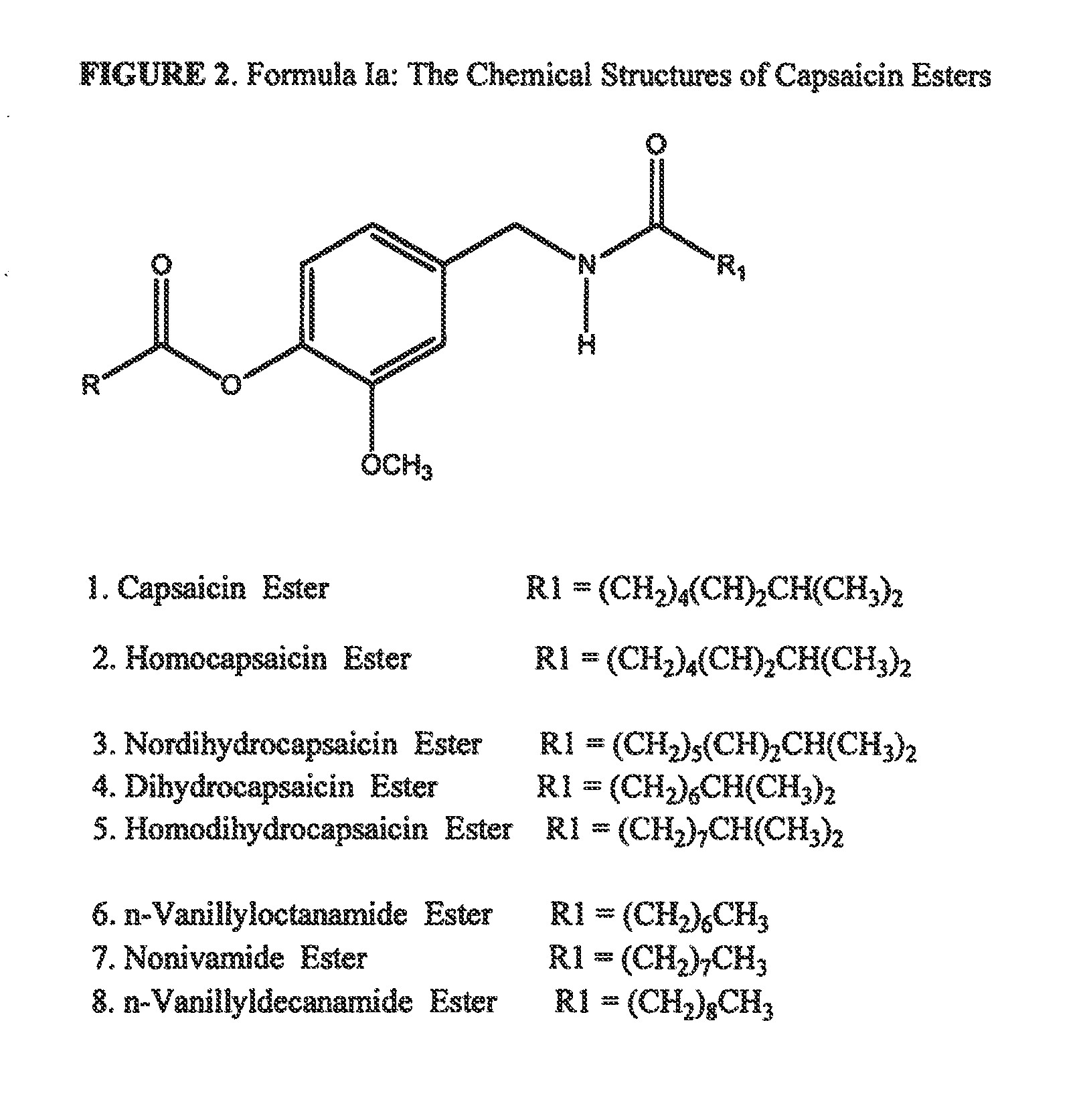
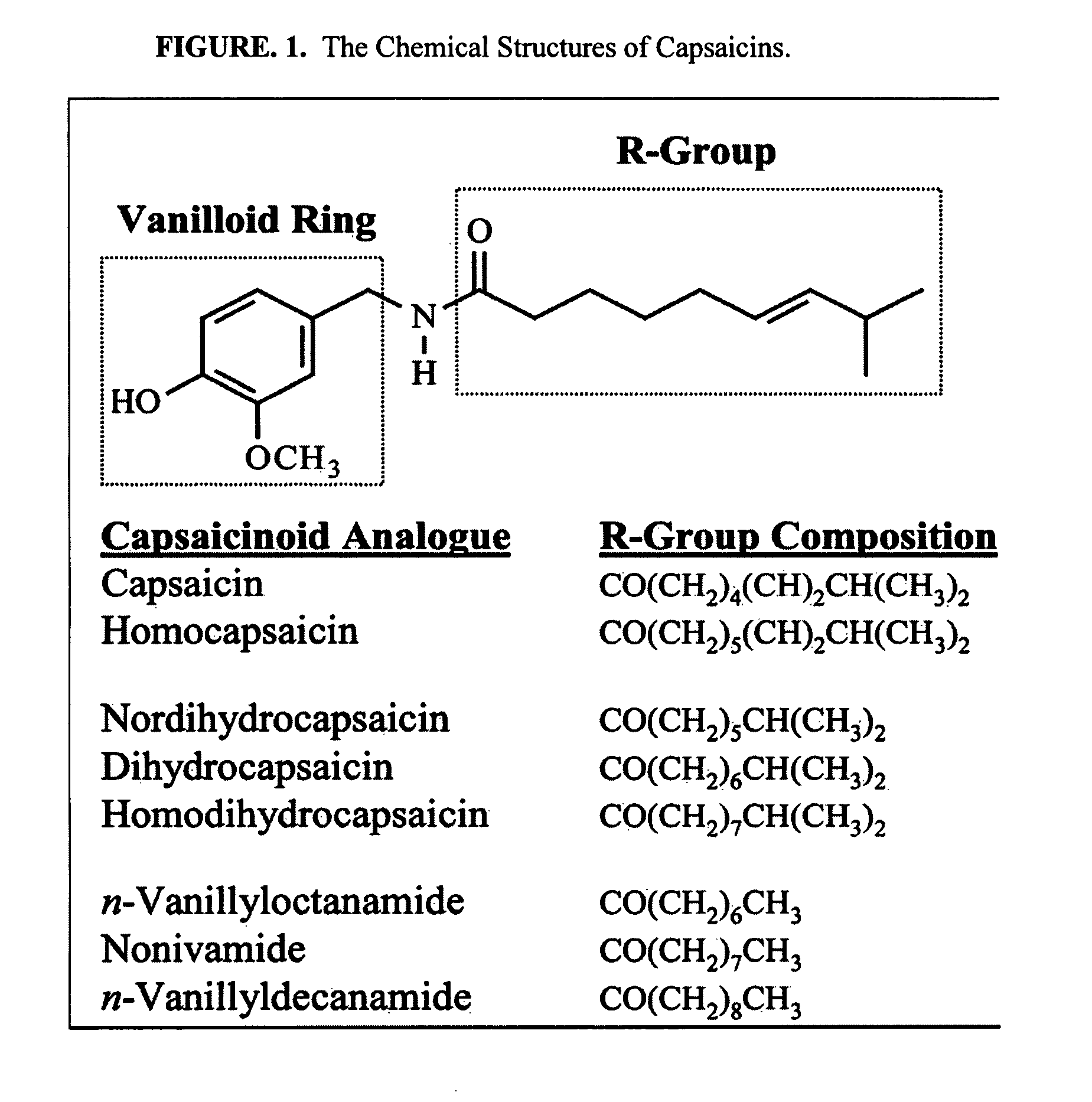
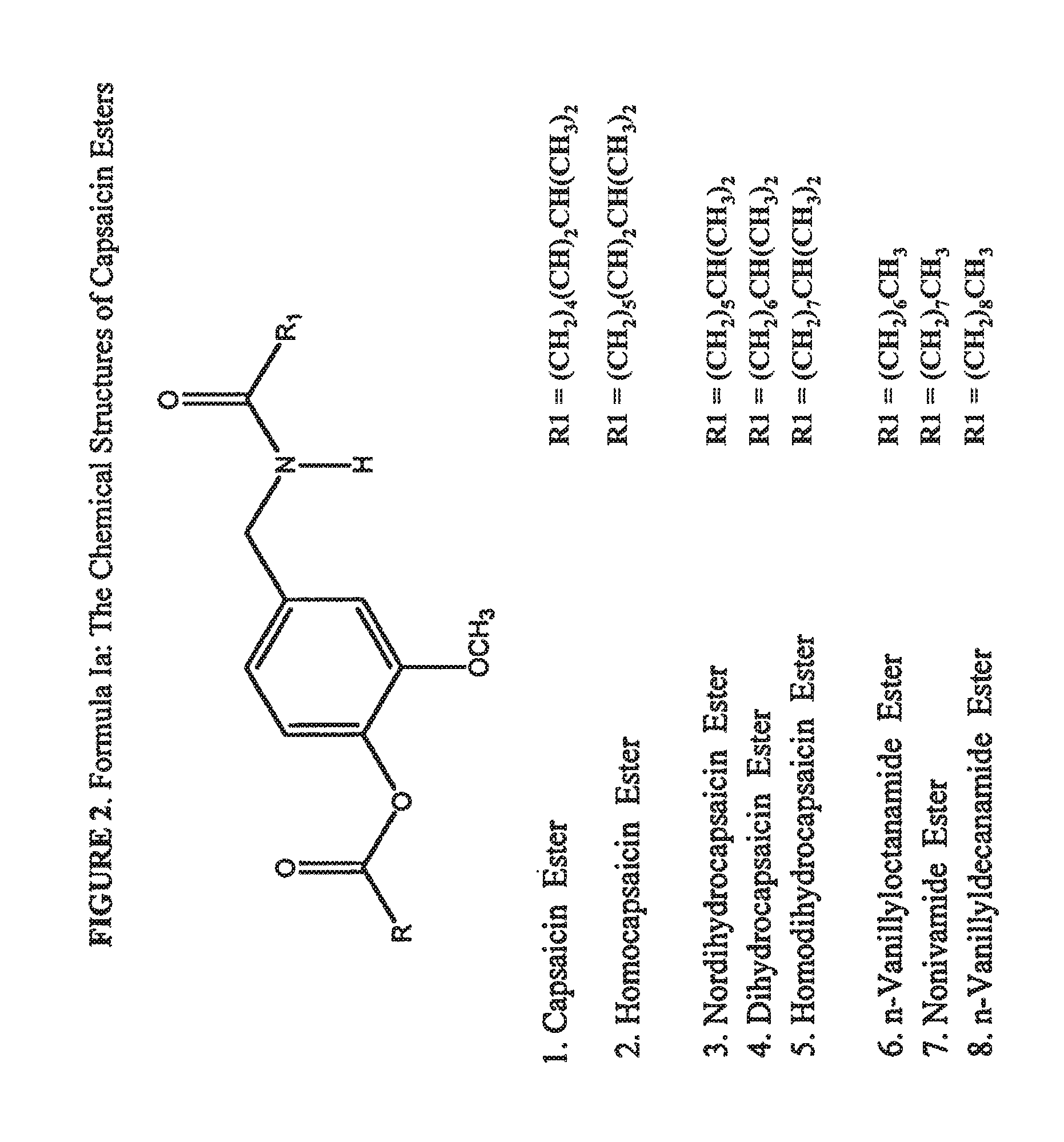
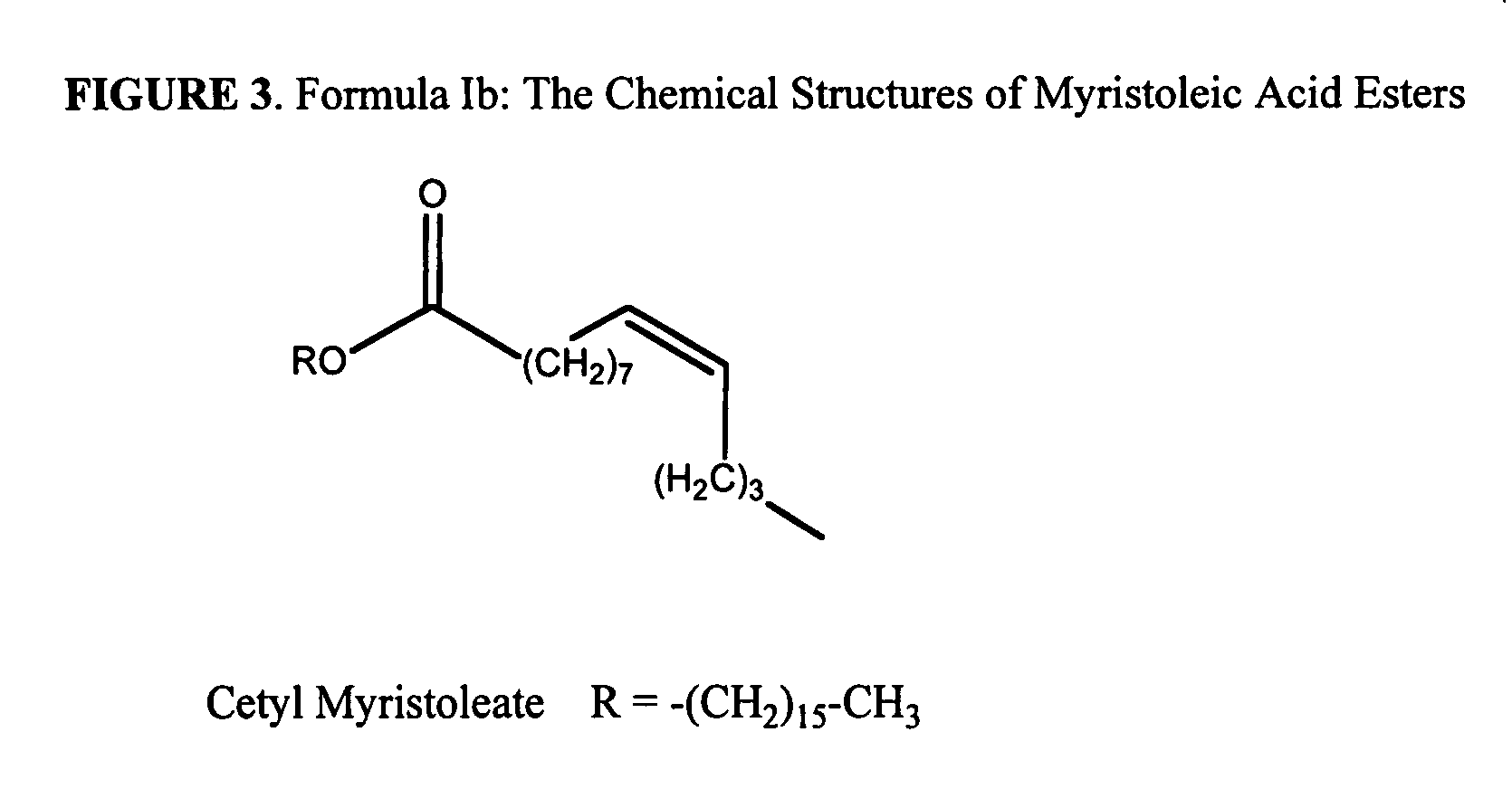
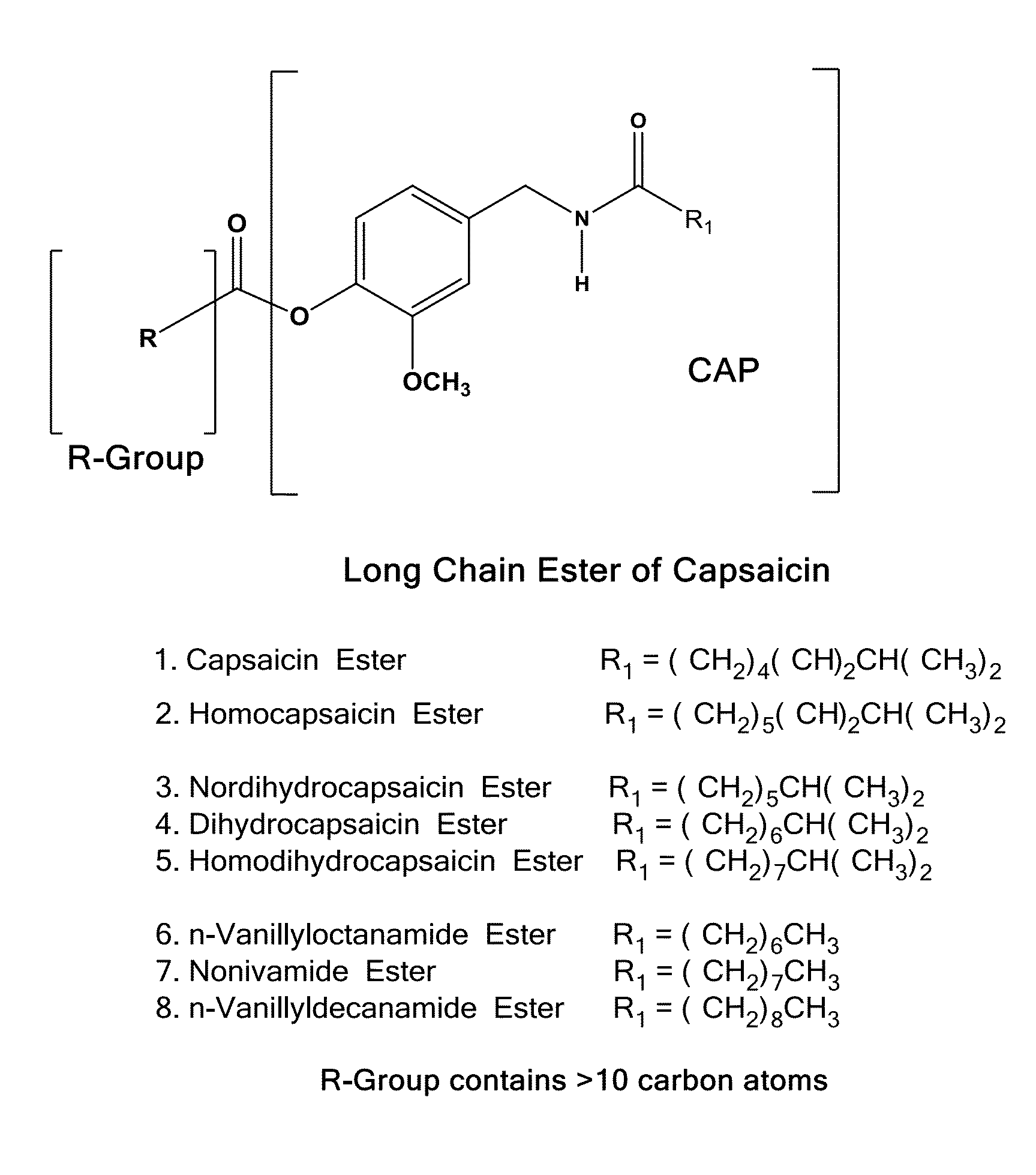
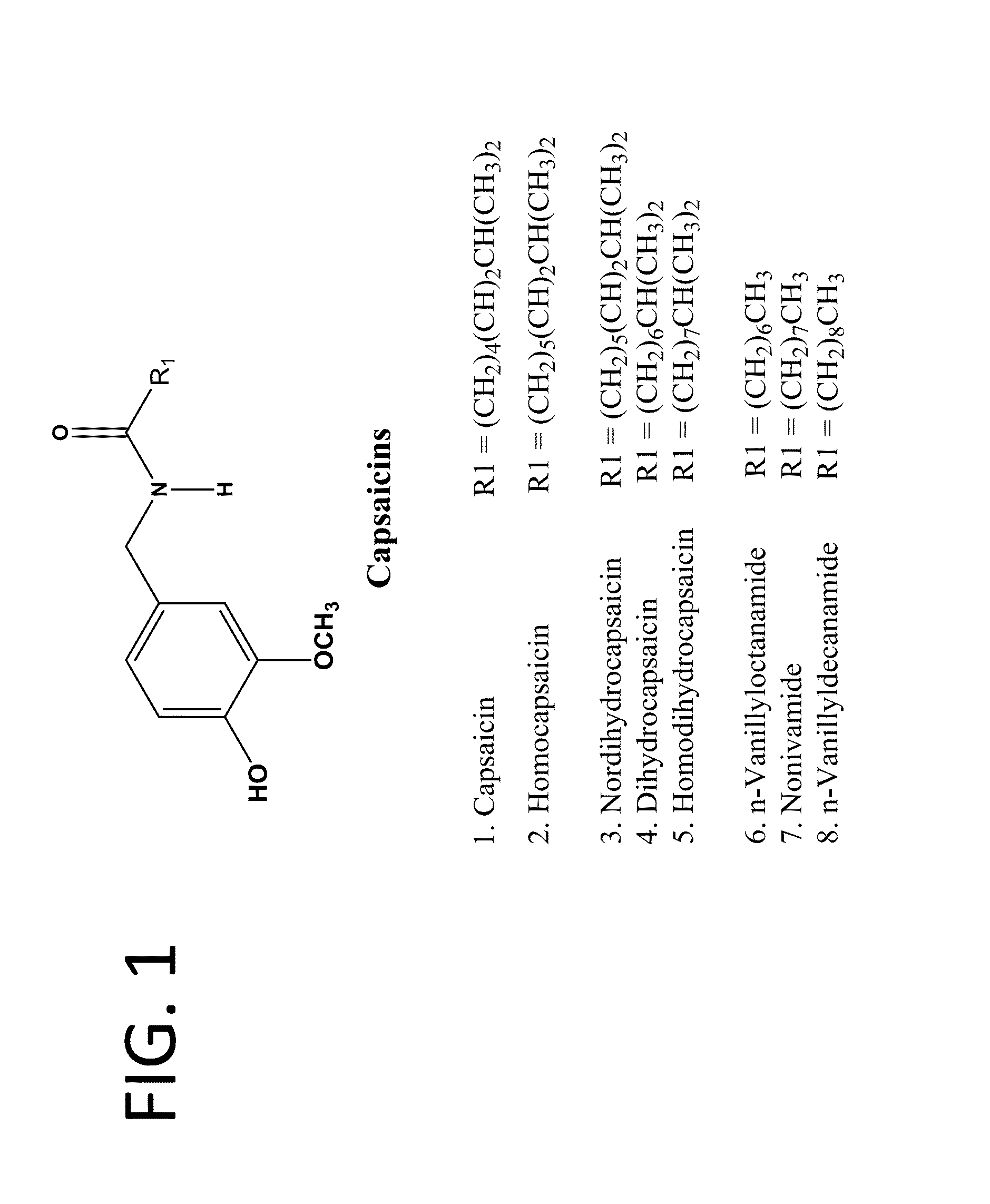
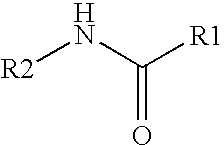
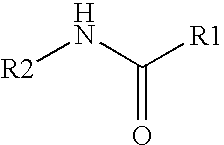
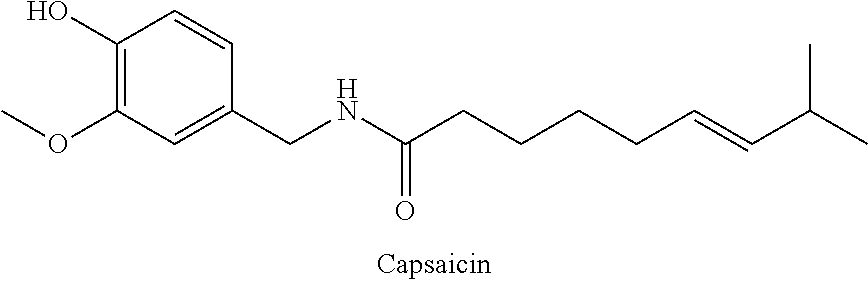
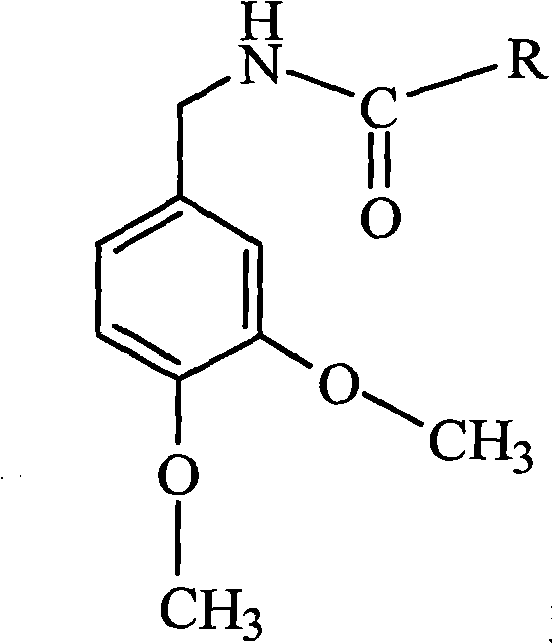
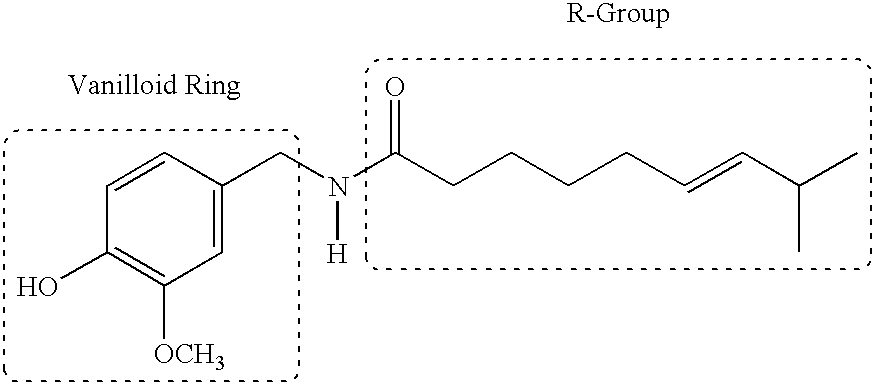
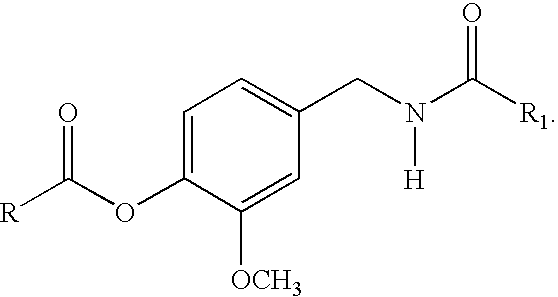
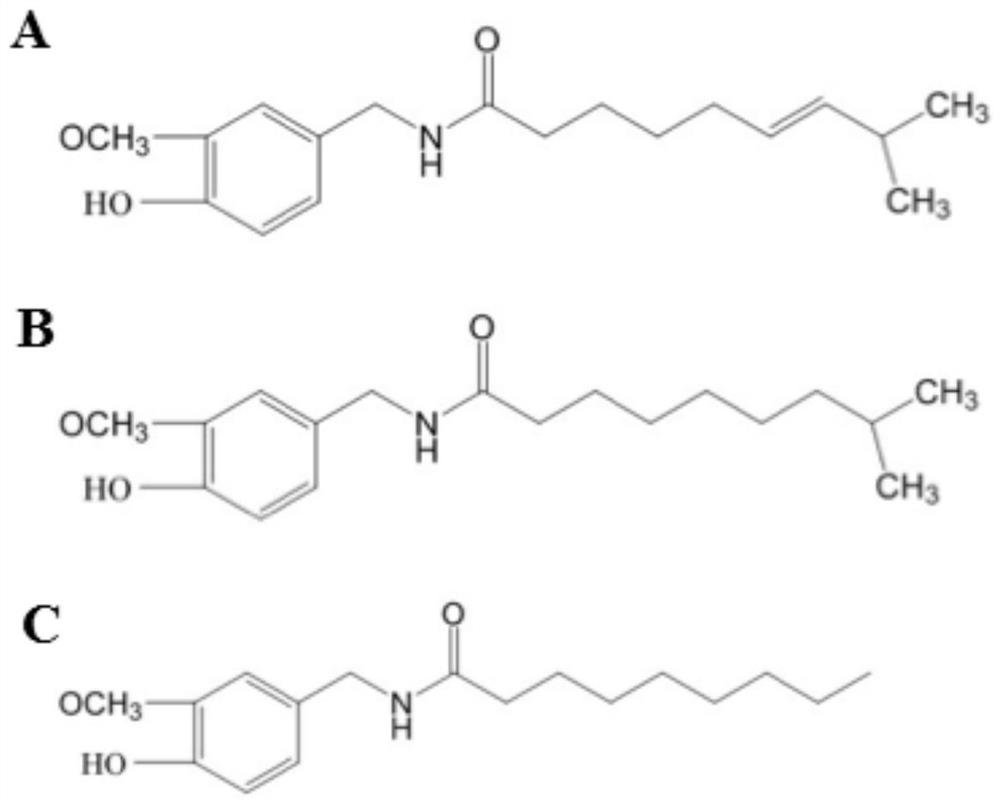
The present invention relates to the formulations of ester derivatives of capsaicin and ester derivatives of myristoleic acid. These derivatives are capable of reverting to the active parent compound following enzymatic or chemical hydrolysis. These derivatives have a higher lipophilicity, lipid solubility and less irritation to the skin than the parent compound, and hence are better able to be incorporated into certain pharmaceutical formulations, including cream and ointment pharmaceutical formulations. The pharmaceutical compositions of the present invention contain a compound of following formula (Ia):R—CO-CAP (Ia)wherein CAP refers to collectively the capsaicins represented in FIG. 1 and a compound of formula (Ib):MCO-O—R (Ib)wherein MCO refers to myristoleic acid.In formulae Ia and Ib, R is selected from alkyl groups of up to about 18 carbon atoms and aryl groups of up to about 18 carbon atoms and alkylene group of up to about 18 carbon atoms and an arylene group of up to about 18 carbon atoms. The alkyl, aryl and alkylene groups may be substituted or un-substituted, branched or straight chains. In addition, R may contain heteroatoms and may be straight chained or branched.The pharmaceutical compositions containing compounds of formulae Ia and Ib are useful for pain management in mammals in vivo and have been contemplated to be used in the treatment of various pains in humans.
Owner:TRINITY LAB INC
Esters of Capsaicin for Treating Pain
InactiveUS20110218180A1Improve lipophilicityNon-irritation to skinAntibacterial agentsBiocideSolubilityIrritation
The present invention relates to the formulations of ester derivatives of capsaicin and ester derivatives of myristoleic acid. These derivatives are capable of reverting to the active parent compound following enzymatic or chemical hydrolysis. These derivatives have a higher lipophilicity, lipid solubility and less irritation to the skin than the parent compound, and hence are better able to be incorporated into certain pharmaceutical formulations, including cream and ointment pharmaceutical formulations. The pharmaceutical compositions of the present invention contain a compound of following formula (Ia):R—CO—CAP (Ia)wherein CAP refers to collectively the capsaicins represented in FIG. 1 and a compound of formula (Ib):MCO—O—R (Ib)wherein MCO refers to myristoleic acid.In formulae Ia and Ib, R is selected from alkyl groups of up to about 18 carbon atoms and aryl groups of up to about 18 carbon atoms and alkylene group of up to about 18 carbon atoms and an arylene group of up to about 18 carbon atoms. The alkyl, aryl and alkylene groups may be substituted or un-substituted, branched or straight chains. In addition, R may contain heteroatoms and may be straight chained or branched.The pharmaceutical compositions containing compounds of formulae Ia and Ib are useful for pain management in mammals in vivo and have been contemplated to be used in the treatment of various pains in humans.
Owner:TRINITY LAB INC
Nutrigenomics methods and compositions
The present invention provides a proprietary compositions and systems to modulate genetic and metabolomic contributing factors affecting disease diagnosis, stratification, and prognosis, as well as the metabolism, efficacy and / or toxicity associated with specific vitamins, minerals, herbal supplements, homeopathic ingredients, and other ingredients for the purposes of customizing a subject's nutritional supplement formulation to optimize specific health outcomes. Specific to this invention the utilization of certain known polymorphic genes associated with Substance Use Disorder (SUD) are analyzed to target certain genetic anomalies that lead to a high risk and predisposition to SUD. The genotypic patterns are then utilized to provide certain nutritional customized solutions especially related to the attenuation of aberrant abuse of physician prescribed narcotic pain medication across all pain conditions. A priority GENOPROFILE is measured and directs the customization of a subsequent nutraceutical to act as a therapeutic modality. Specifically the treatment includes slow attenuation of the pain medication by incorporating orals (shakes, liquid beverages, pills, tablets, troche, ointments etc.), Intramuscular, Intravenous, intra-rectal and any form necessary to deliver a sufficient amount of an anti-craving and anti-stress nutraceutical. Moreover, the invention includes examples of novel analgesic ointments coupling Synaptamine and such analgesic and other anesthetic compounds including but not limited to Gabapentin, Ketamine, Baclofen, Ketoprofen, Amitriptyline, Lidocaine, Cyclobenzapine, Diclofenac, Menthol, Camphor and Capsaicin. The GENOPROFILE will be used to determine pain sensitivity Intolerance.
Owner:BLUM KENNETH +3
Methods and compositions for administration of TRPV1 agonists
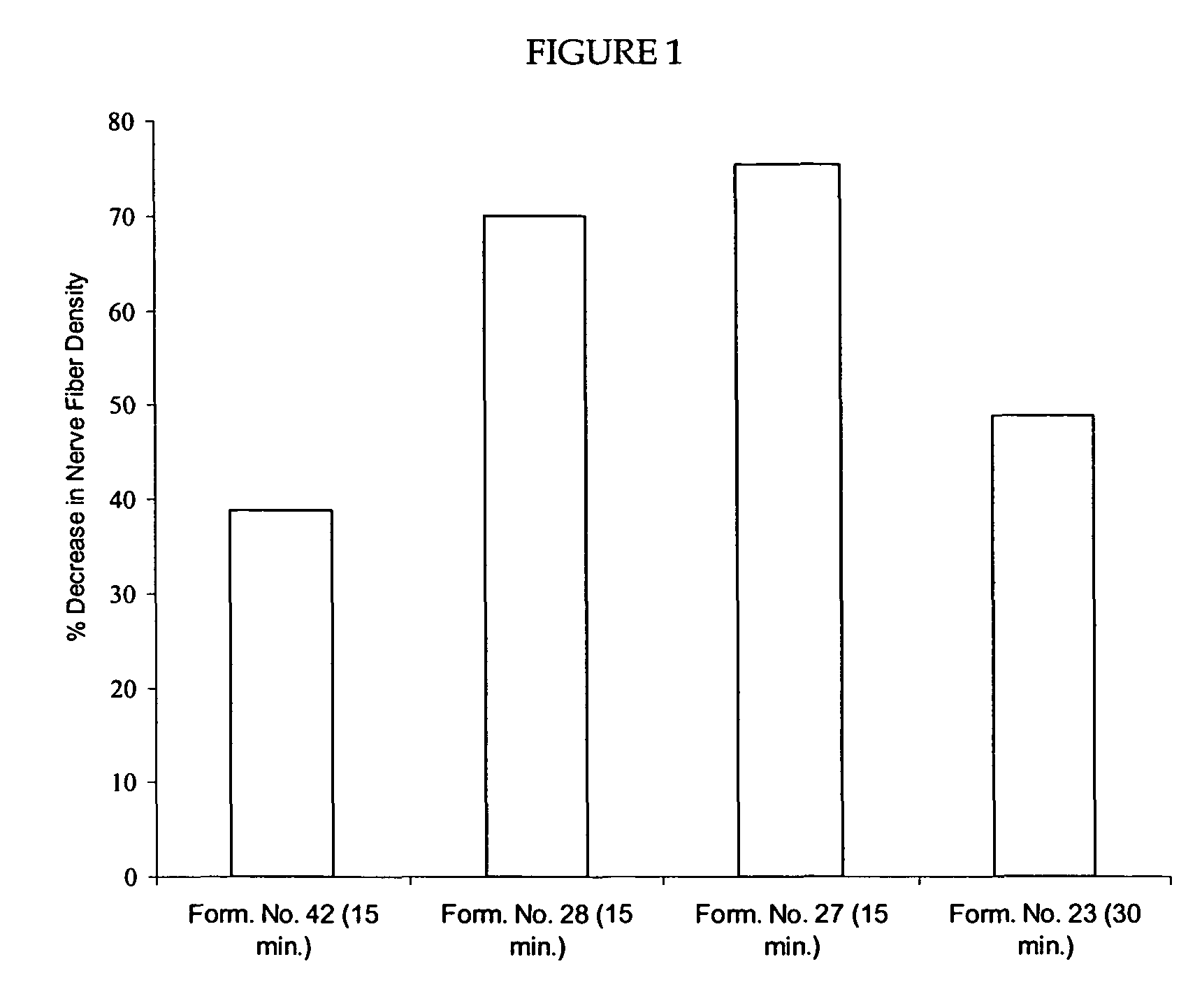
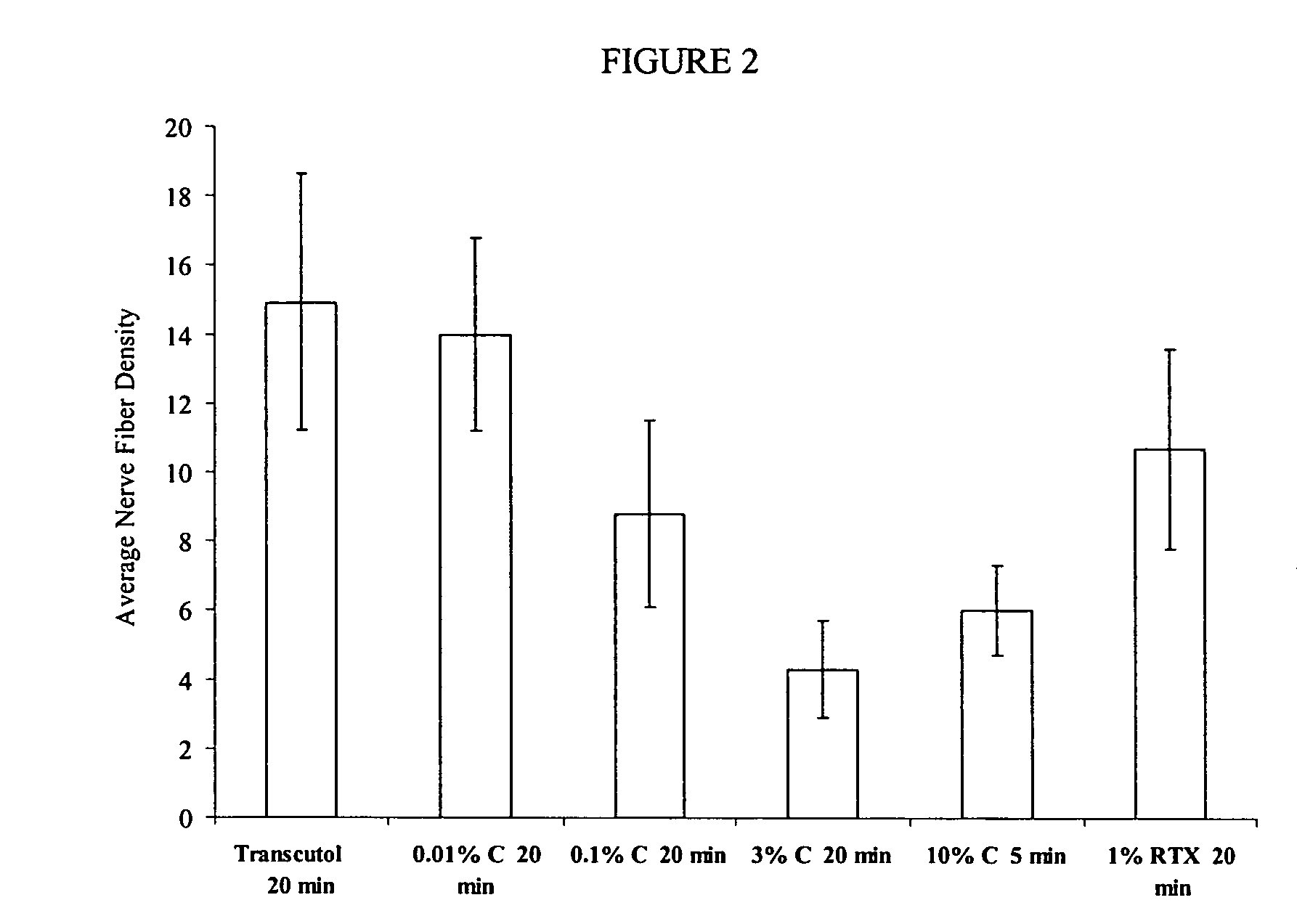
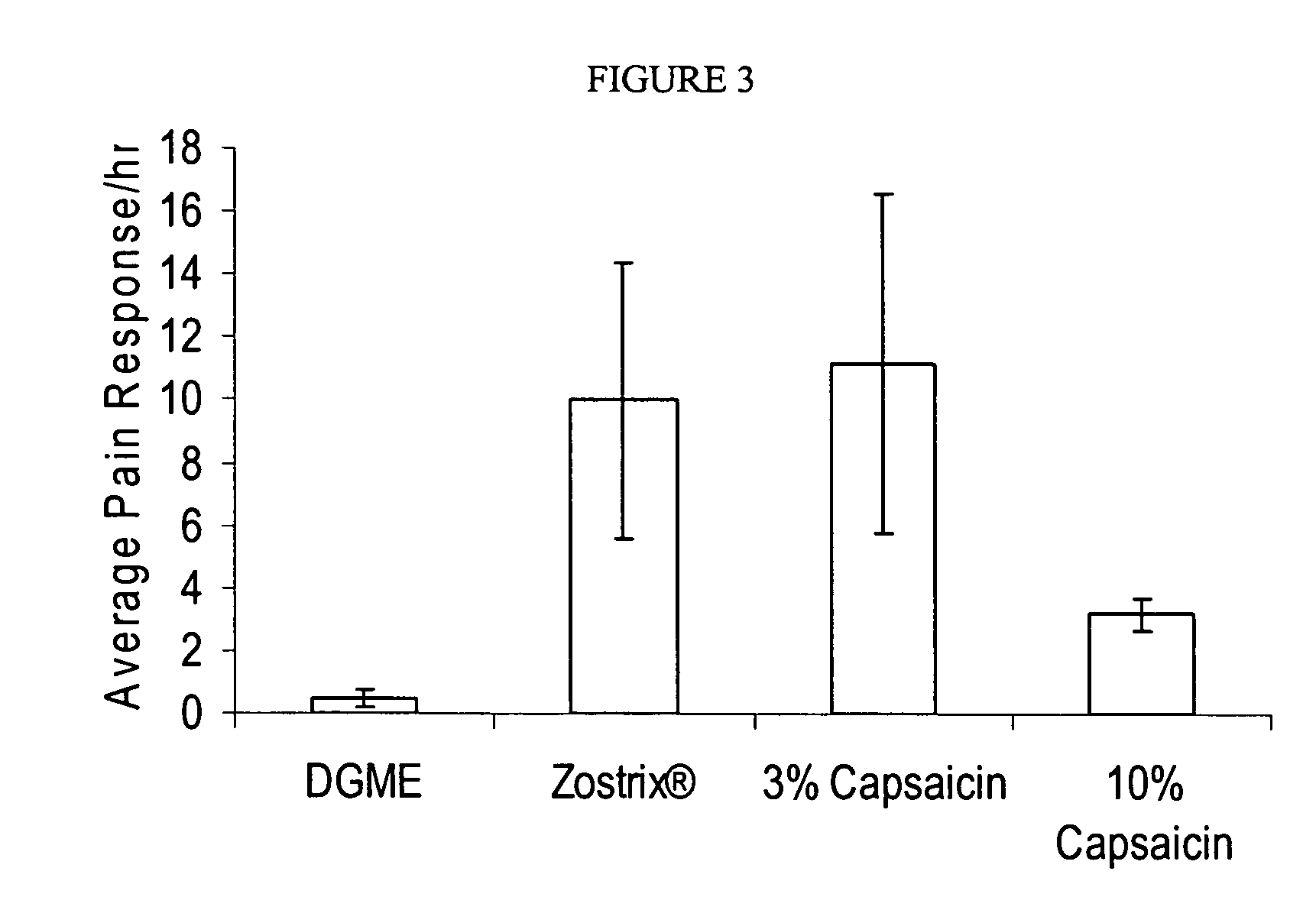
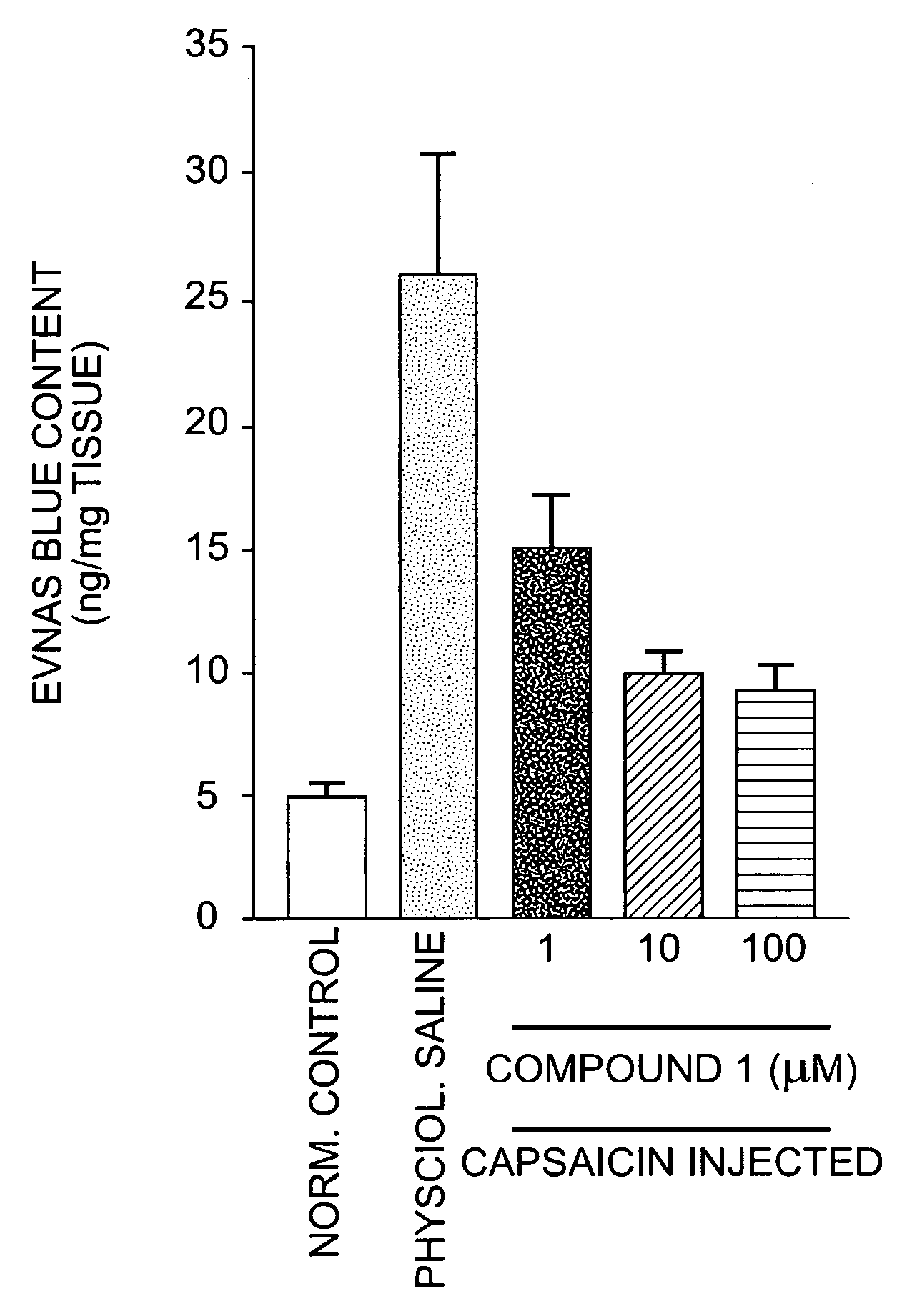
Compositions are provided that contain a TRPV1 agonist, such as capsaicin, and a solvent system. Topical application of the composition results in rapid delivery of agonist to the dermis and epidermis. Method of using the compositions for reducing nociceptive nerve fiber function in subjects, and for treatment of capsaicin-responsive conditions are also provided.
Owner:GRT US HLDG INC
Esters of capsaicin for treating pain
ActiveUS7943666B2Improve lipophilicityNon-irritation to skinAntibacterial agentsBiocideSolubilityIrritation
The present invention relates to the formulations of ester derivatives of capsaicin and ester derivatives of myristoleic acid. These derivatives are capable of reverting to the active parent compound following enzymatic or chemical hydrolysis. These derivatives have a higher lipophilicity, lipid solubility and less irritation to the skin than the parent compound, and hence are better able to be incorporated into certain pharmaceutical formulations, including cream and ointment pharmaceutical formulations. The pharmaceutical compositions of the present invention contain a compound of following formula (Ia):R—CO-CAP (Ia)wherein CAP refers to collectively the capsaicins represented in FIG. 1 and a compound of formula (Ib):MCO-O—R (Ib)wherein MCO refers to myristoleic acid.In formulae Ia and Ib, R is selected from alkyl groups of up to about 18 carbon atoms and aryl groups of up to about 18 carbon atoms and alkylene group of up to about 18 carbon atoms and an arylene group of up to about 18 carbon atoms. The alkyl, aryl and alkylene groups may be substituted or un-substituted, branched or straight chains. In addition, R may contain heteroatoms and may be straight chained or branched.The pharmaceutical compositions containing compounds of formulae Ia and Ib are useful for pain management in mammals in vivo and have been contemplated to be used in the treatment of various pains in humans.
Owner:TRINITY LAB INC
Capsaicin antifouling paint
InactiveCN1709997AHigh mechanical strengthStay alkaline for a long timeCoatingsCalcium hydroxideSodium Bentonite
The invention relates to a kind of capsicum alkali antifouling dope. Its materials weight proportions are: active acrylic resin 18-25%, calcareousness powderó±30-40%, calcareousness powderó�18-30%, calcium hydroxide 2-6%, capsicum alkali 0.05-0.10%, bentonite 9-14% and silica powder. It is a kind of antifouling dope which prevents sea biology from accreting with active acrylic resin as basic material, calcareousness powder and calcium hydroxide of different granularities, and capsicum alkali as driving agent. The quantity average molecular weight of active acrylic resin Mn = P800, the weight average weight Mw = 17100, and the dispersing intensity D = 1.75. Through seawater (pH = 7.5) simulating hanging board experimentation, the seeping rate of capsicum alkali of the dope stabilizes at 74 - 118 ª–gíñm-2d-1, its pH stabilizes at 9.5 - 10.0, and its effective antifouling period can get to over 3 years. The dope of the invention is non-toxic, highly effective, and low-cost.
Owner:心脑合一(上海)教育科技有限公司
Pharmaceutical Compositions Comprising Capsaicin Esters for Treating Pain and Cold Sores
InactiveUS20140134261A1Increase heart rateGood blood pressureBiocideHydroxy compound active ingredientsDiseaseAmyris
The present invention relates to pharmaceutical compositions comprising ester(s) of capsaicin and at least one other agent selected from salicylates, menthol, boswellic acids, DMSO, methyl sulfonylmethane, NSAIDs, corticosteroids, emu oil, opioid agonists and antagonists, NMDA antagonists, tramadol, hyaluronic acid, α2δ ligands, santalol, santalyl acetate, amyris alcohol, amyris acetate, aloe vera gel and aloe vera juice, for improved therapeutic properties. Further, the present invention relates to pharmaceutical compositions comprising high concentrations of ester(s) of capsaicin. Further, the present invention relates to a method of relieving pain due to various diseases in subjects by administering the pharmaceutical compositions of the invention. Further, the present invention relates to methods of relieving fever blisters due to cold sores in subjects by administering the pharmaceutical compositions comprising an ester of capsaicin and one other agent selected from santalol, santalyl acetate, amyris alcohol and amyris acetate.
Owner:TRINITY LAB INC
Use of vanillin receptor agonist in preparation of product for resisting Alzheimer disease
InactiveCN1736485ATechnical expertiseEnhance pharmacological effectsNervous disorderHeterocyclic compound active ingredientsCapsaicinAlzheimer's disease
Owner:SHANGHAI MEDICILON INC
Dermal compositions of substituted amides and the use thereof as medication for pain and pruritus
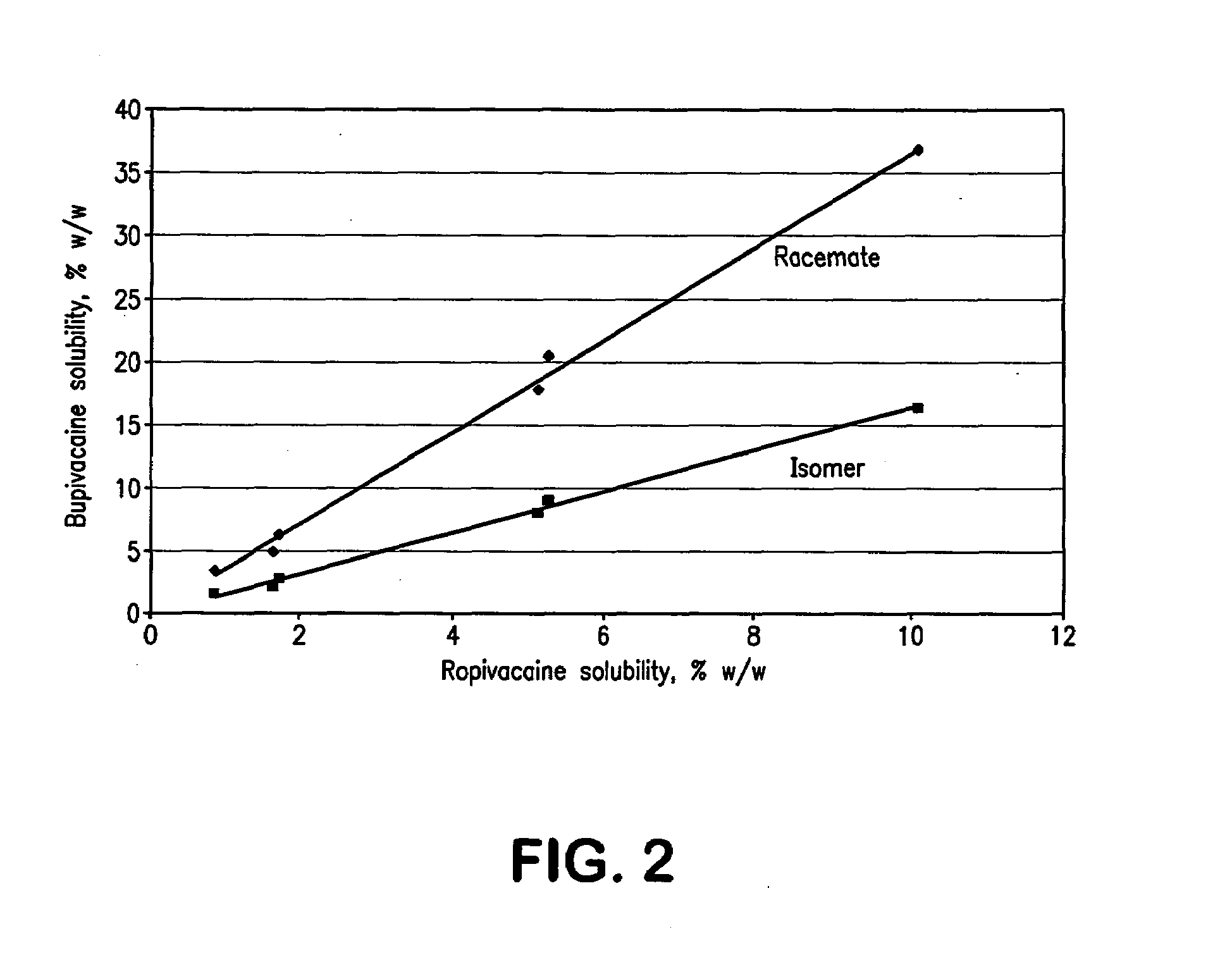
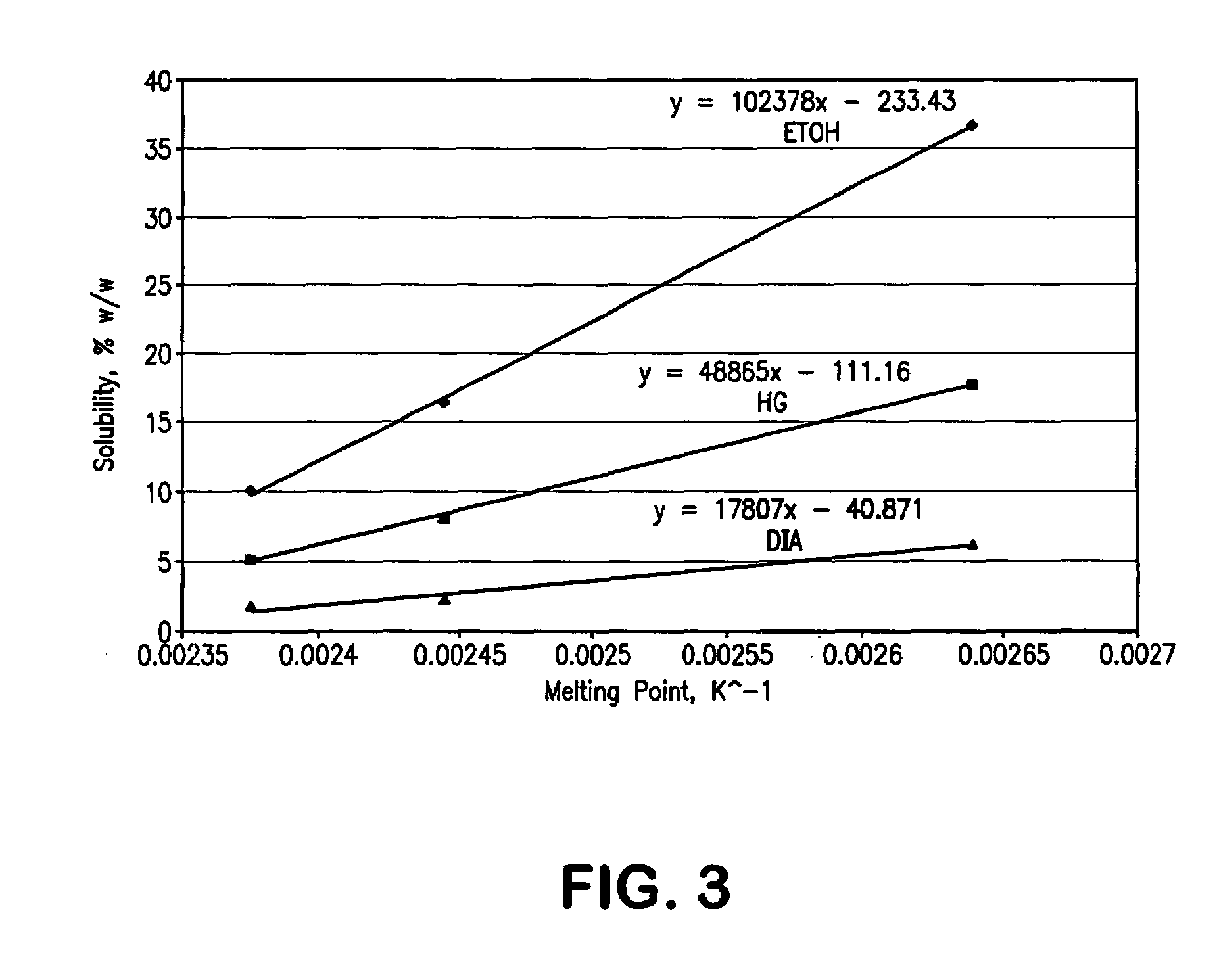
Dermal compositions comprising topical formulations of bupivacaine or ropivacaine, characterized by effective dermal absorption and long duration of dermal anesthetic activity, and intended for use in patients suffering from pruritus and dermal pain, including neuropathic pain, are provided. Compositions containing both bupivacaine and capsaicin are provided. Methods of alleviating pain by the topically administration of these compounds are also provided.
Owner:BRIDGE PHARMA INC
Use of Hypothermia Inducing Drugs
The present invention relates to the induction of hypothermia in humans in a predictable and dose responsive fashion by use of a pharmaceutical composition comprising a vanilloid receptor agonists, capsaicinoid or capsaicinoid-like agonist capable of inducing hypothermia, thereby benefiting patients suffering from illnesses characterized by tissue anoxia.
Owner:HJERTEFORSKNINGSFONDEN
Method for separating and purifying capsaicin and paprika red pigment through molecular distillation
InactiveCN103232726AReduce pollutionKeep naturalAlkaloids chemistryAlkaloidsDihydrocapsaicinPaprika oleoresin
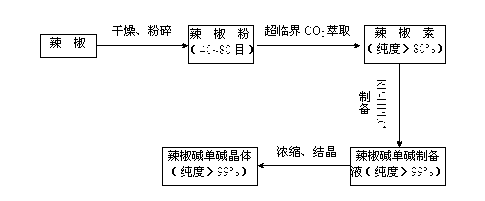
The invention relates to the field of separation and purification of capsaicin and paprika red pigment, and particularly discloses a method for preparing paprika red pigment and a capsaicin material. According to the method, capsicum oleoresin is subjected to three-level molecular distillation to respectively obtain the capsaicin material and the paprika red pigment, wherein the total content of capsaicin and dihydrocapsaicin in the capsaicin material is greater than 70%, and the color value of the paprika red pigment is higher than 200. The capsaicin material is recrystallized and purified, the total content of capsaicin and dihydrocapsaicin in crystallization is more than 85%, further capsaicin crystals and dihydrocapsaicin crystals are obtained through preparation and separation of high performance liquid chromatography, and the content of each of the capsaicin crystals and the dihydrocapsaicin crystals is more than 95%. The method is simple and convenient to operate and low in production cost, has a good separation effect and is suitable for industrial production; and the content of the products is high.
Owner:SUN YAT SEN UNIV
Treatment of hepatic encephalopathy and liver cirrhosis
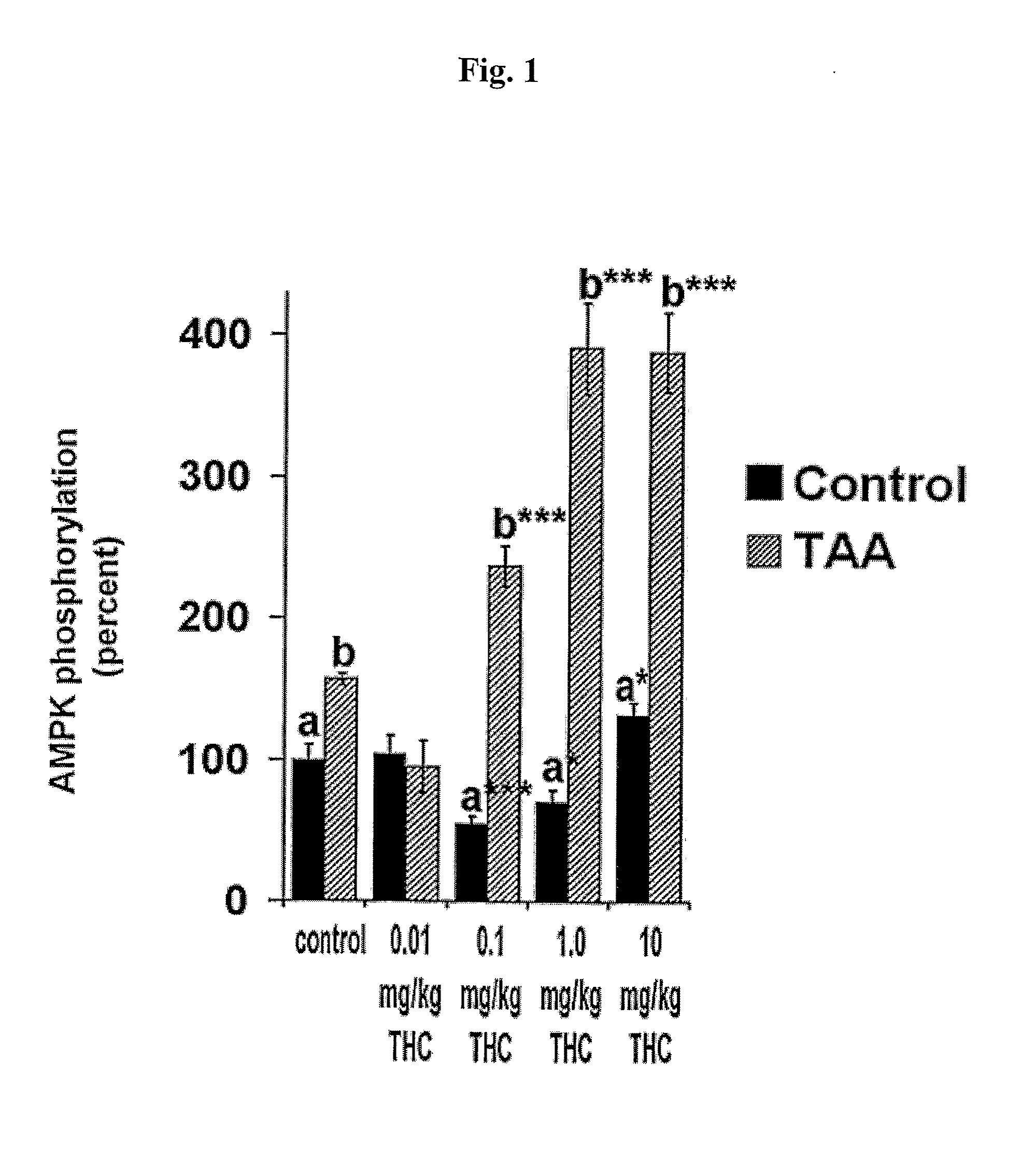
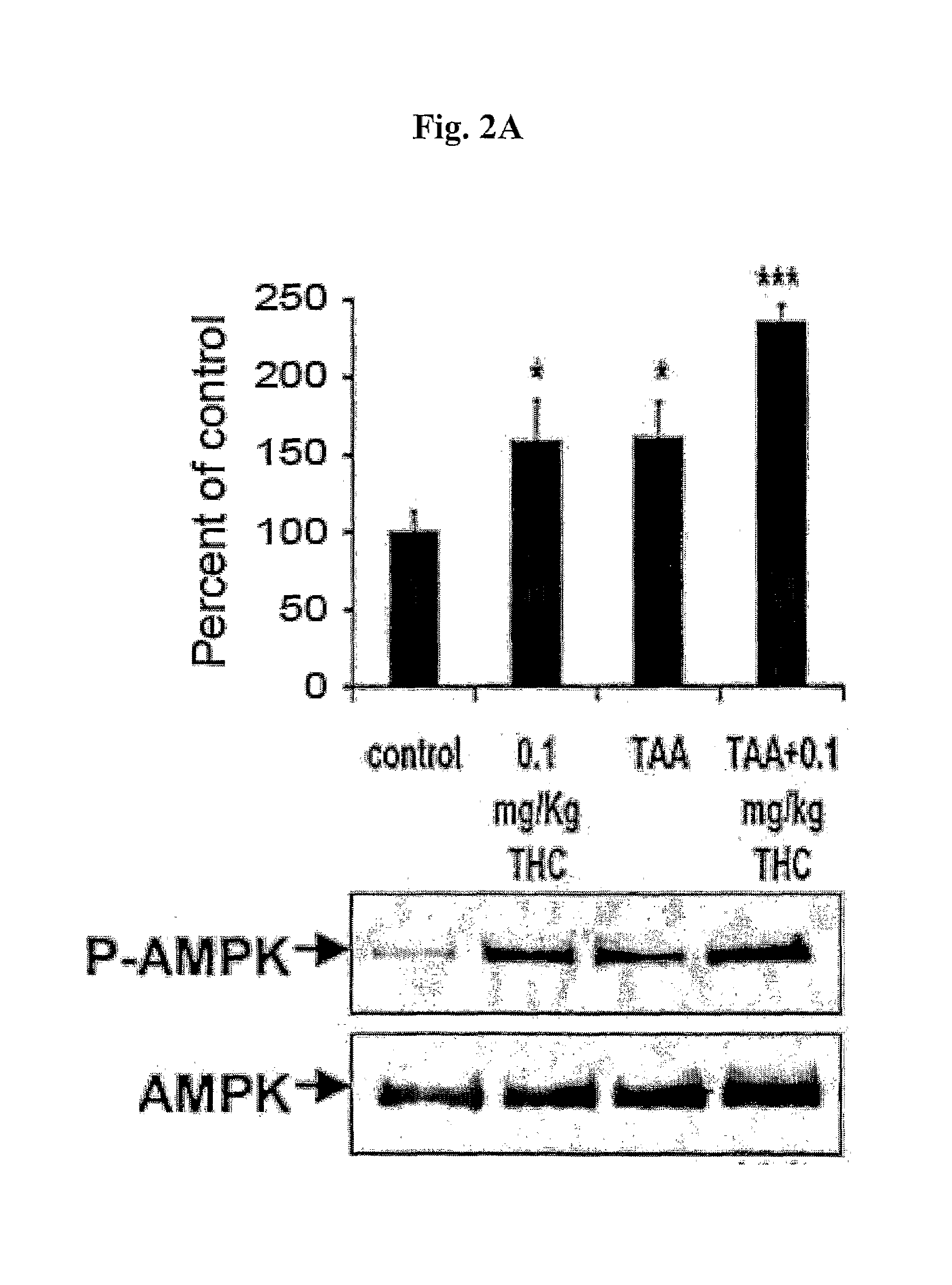
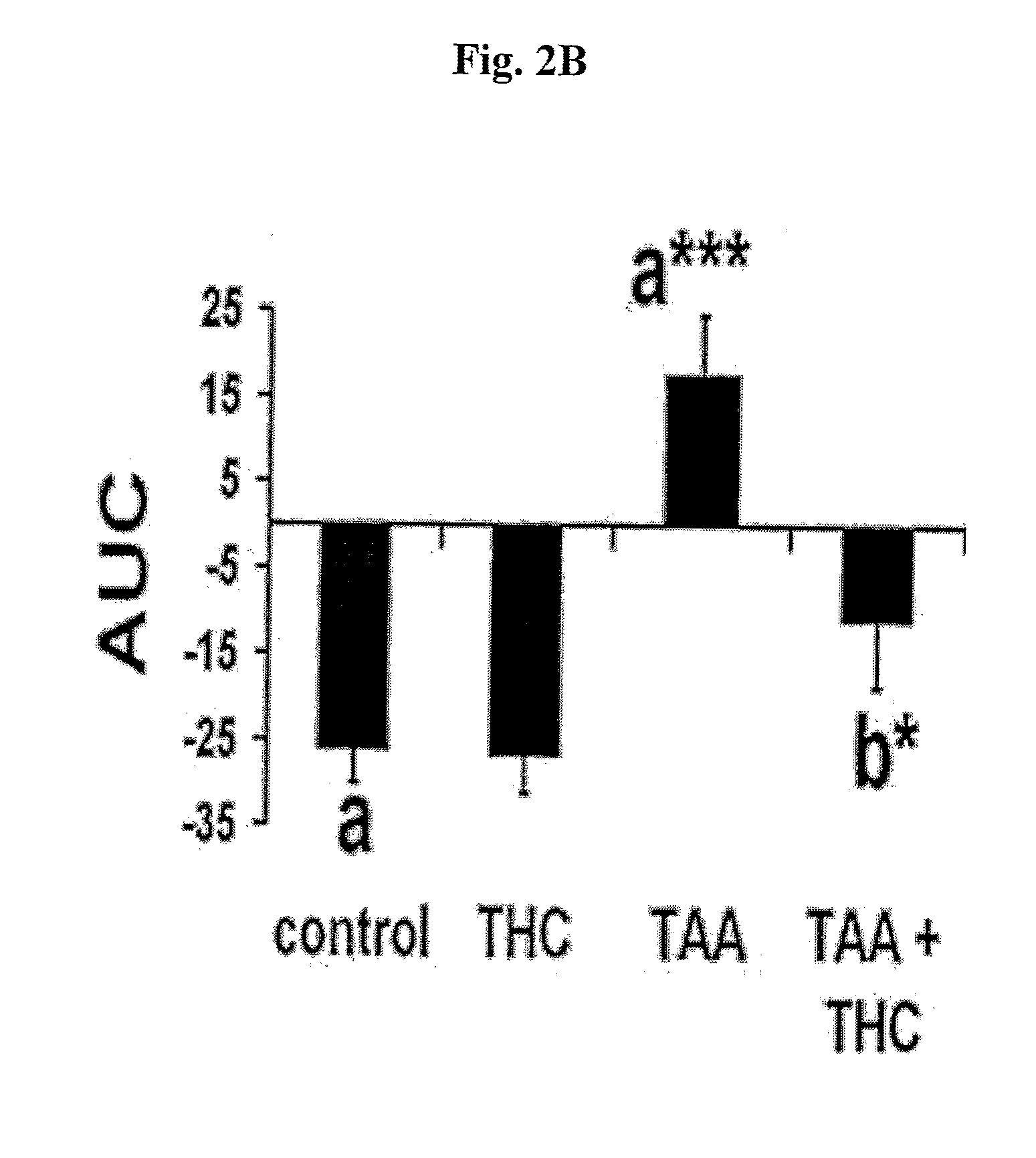
InactiveUS20100022631A1Reduce severityImprove cognitive functionBiocideHydroxy compound active ingredients2-ArachidonoylglycerolCapsaicin
The compounds D9-tetrahydrocannabinol (THC), cannabidiol (CBD) and capsaicin are useful for prevention, treatment, or both, of hepatic encephalopathy. The compounds capsaicin, 2-arachidonoylglycerol (2-AG), HU-308 and cannabidiol are useful for prevention, treatment, or both, of liver cirrhosis.
Owner:YISSUM RES DEV CO OF THE HEBREWUNIVERSITY OF JERUSALEM LTD +2
Production process of capsaicin
InactiveCN103254092ACarboxylic acid amide separation/purificationNatural dyesDihydrocapsaicinFluid phase
The invention relates to a preparation method of an active ingredient in chili and particularly relates to a production process of capsaicin. According to the production process disclosed by the invention, a supercritical fluid extraction method is adopted for separating capsaicin and capsanthin, and a high performance liquid chromatography is further used for performing preparation on a crude extract to obtain high-purity nordi-hydrocapsaicin, capsaicin and dihydrocapsaicin. The production process disclosed by the invention has the advantages of quickness, high efficient and energy conservation and can be industrially popularized.
Owner:CHANGZHOU ADAM BIOTECH
Preparation method of capsicine micro spheres
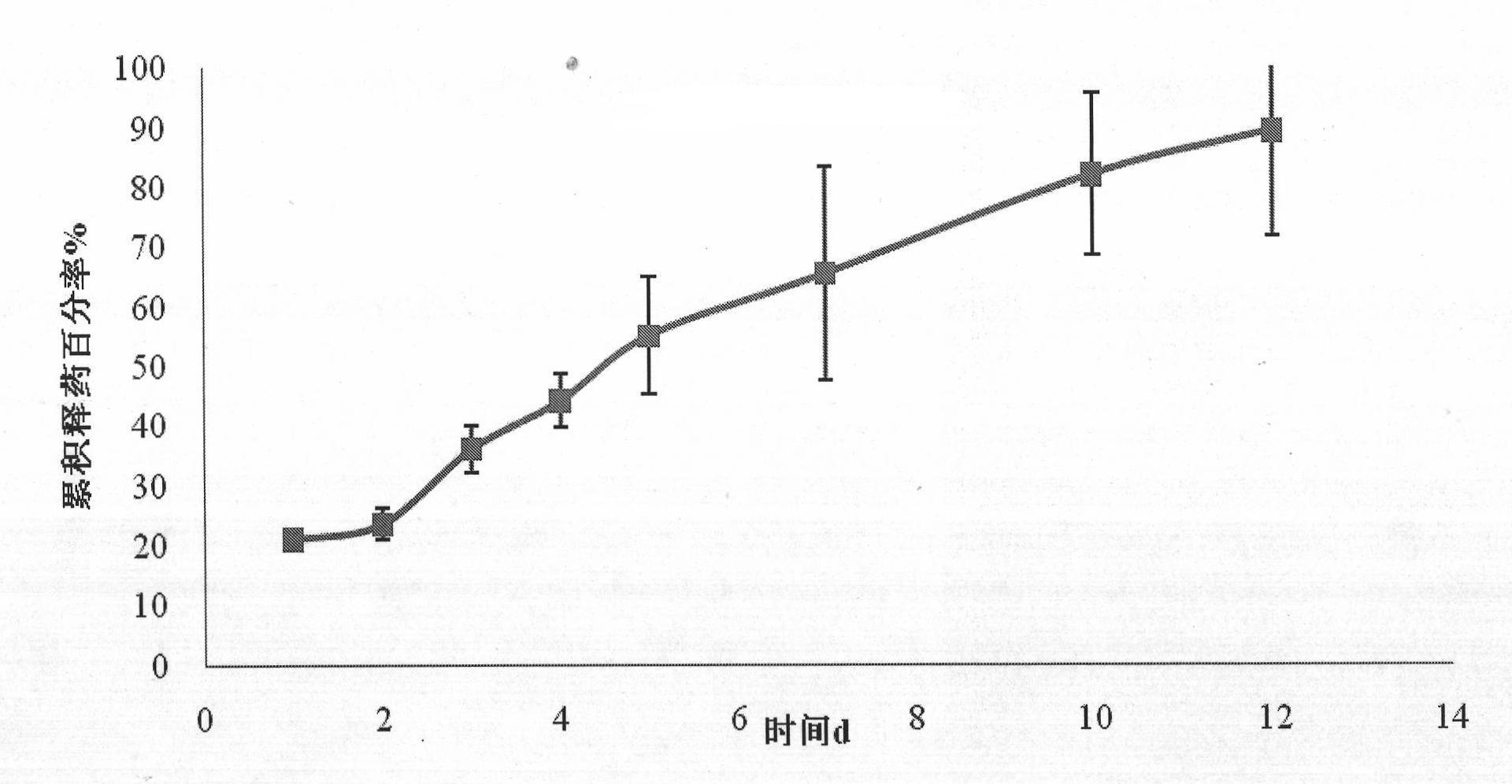
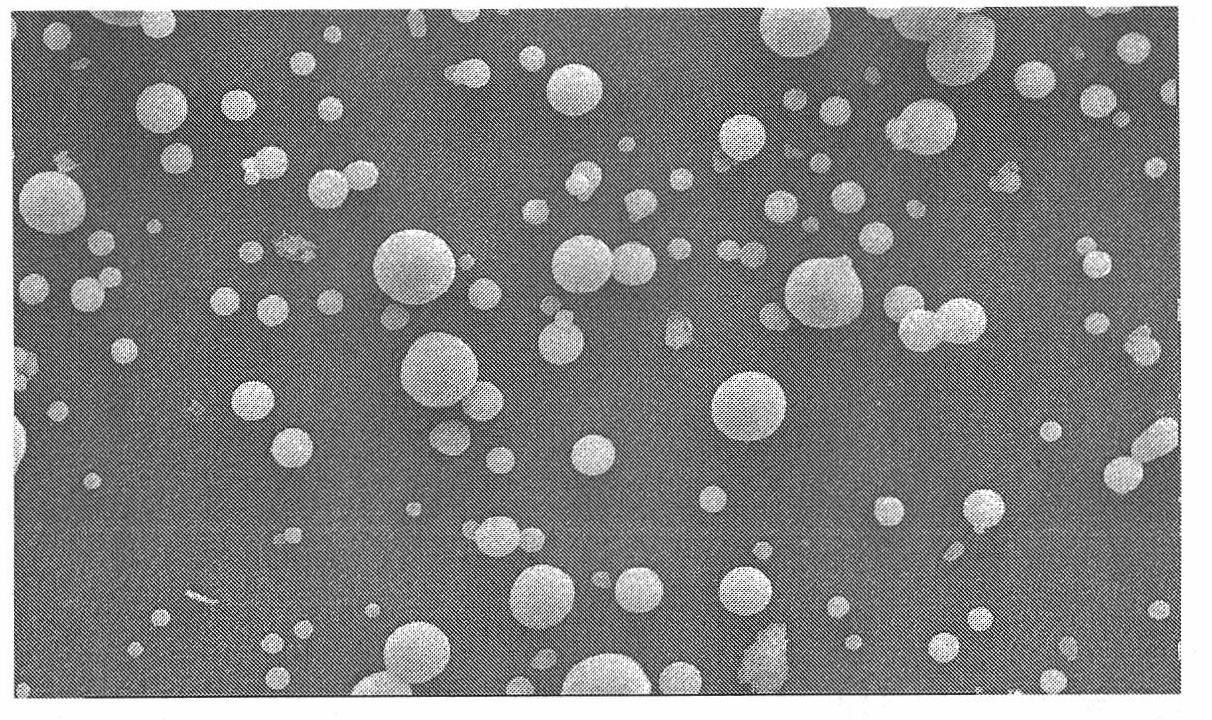
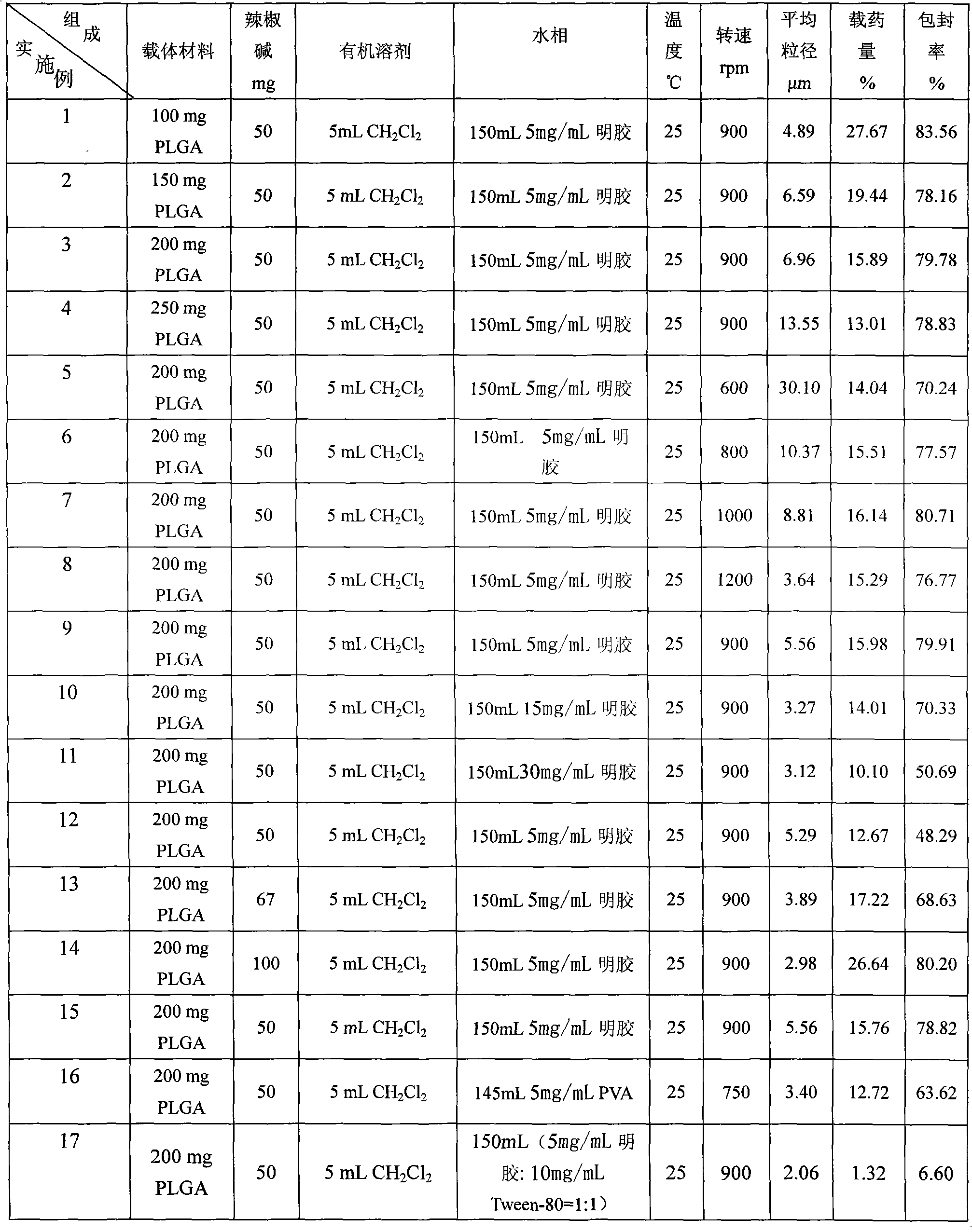
InactiveCN101773475AUnique analgesic effectUnique mechanism of actionOrganic active ingredientsNervous disorderSide effectOrganic solvent
The invention discloses a preparation method of capsicine micro spheres, which comprises the following steps: (1) dissolving capsicine and carrier materials into an organic solvent to obtain an oil phase according to the mixing ratio of 100 to 250 mg / 27 to 100 mg / 2 to 5 mL; (2) dissolving emulsifying agents into water as a water phase, wherein the concentration of the emulsifying agents in the water phase is between 5 and 30 mg / mL; and (3) adding the oil phase into the water phase for forming oil-in-water latex emulsion according to the volume ratio of 1 / 20 to 1 / 40 under the conditions of the temperature between 20 and 30 DEG C and the stirring speed between 500 and 1200 rpm, continuously stirring the oil-in-water latex emulsion for volatilizing the organic solvent, carrying out centrifugation for collecting micro spheres, washing the micro spheres by distilled water, and preparing the capsicine micro spheres through freeze drying. The capsicine micro spheres prepared by the method of the invention can be made into various slow-release preparations, the stimulation of the capsicine can be reduced, and the medicine concentration can be maintained for a long time, so the medication times can be reduced, the toxic and side effect can be reduced, and the adaptability of patients can be improved.
Owner:LOGISTICS UNIV OF CAPF
Pharmaceutical composition for therapy of interstitial cystitis
ActiveUS7335668B2Increase painSymptoms improvedBiocideNervous disorderInterstitial cystitisDepressant
A depressant of capsaicin-sensitive sensory nerve, containing quinuclidin-3′-yl 1-phenyl-1,2,3,4-tetrahydroisoquinoline-2-carboxylate or a salt thereof as an active ingredient, specifically a therapeutic drug of interstitial cystitis, hypersensitive disorder of the lower urinary tract, and / or abacterial prostatitis.
Owner:ASTELLAS PHARMA INC
Hair growth liquid composition for treating alopecia seborrhoeica and preparation method of hair growth liquid composition
InactiveCN106880693AGood for transdermal penetrationGood for hair loss preventionHydroxy compound active ingredientsPharmaceutical delivery mechanismSophocarpidineApigenin
The invention discloses a hair growth liquid composition for treating alopecia seborrhoeica. The hair growth liquid composition is mainly prepared from the following components in parts by weight: 1-20 parts of glycyrrhizic acid, 1-20 parts of sophocarpidine, 1-20 parts of tanshinone, 1-20 parts of oleanolic acid, 1-20 parts of ginsenoside, 1-20 parts of capsaicin, 1-20 parts of apigenin and 5-50 parts of menthol. A hair growth liquid is prepared by a dissolution method; the used solvent is medical alcohol and the alcohol concentration is 15-95%; and the hair growth liquid composition is simple in preparation technology and low in cost. The hair growth liquid has an obvious curative effect on alopecia seborrhoeica, is convenient to use and free of toxic or side effect and has a wide market prospect.
Owner:广州国草夏方生物科技有限公司
Nucleic acid sequences encoding capsaicin receptor and capsaicin receptor-related polypeptides and uses thereof
The present invention features vanilloid receptor polypeptides and vanilloid receptor-related polypeptides, specifically the capsaicin receptor subtypes VR1 and VR2 (VRRP-1), as well as the encoding polynucleotide sequences. In related aspects the invention features expression vectors and host cells comprising such polynucleotides. In other related aspects, the invention features transgenic animals having altered capsaicin receptor expression, due to, for example, the presence of an exogenous wild-type or modified capsaicin receptor-encoding polynucleotide sequence. The present invention also relates to antibodies that bind specifically to a capsaicin receptor polypeptide, and methods for producing these polypeptides. Further, the invention provides methods for using capsaicin receptor, including methods for screening candidate agents for activity as agonists or antagonists of capsaicin receptor activity, as well as assays to determine the amount of a capsaicin receptor-activating agent in a sample. In other related aspects, the invention provides methods for the use of the capsaicin receptor for the diagnosis and treatment of human disease and painful syndromes.
Owner:RGT UNIV OF CALIFORNIA
Capsaicinoids and uses thereof as medicaments
The present invention is directed towards a medicament comprising a capsaicinoid possessing antiviral activity and useful in the treatment of viral infections and in the treatment of HIV / AIDS. The medicament also possesses neuroplasticity-enhancing activity and is useful in the treatment of stress and other psychological conditions. Further, the medicament possesses anti-inflammatory activity and is useful in the treatment of arthritis. The medicament has also been shown to possess antibacterial activity.
Owner:MUZARI MANDISHORA ISRAEL
Method for synthesizing N-(3,4-dimethoxybenzyl)amide capsaicine homologous compounds
InactiveCN102001958ASimple processRaw materials are easy to getOrganic compound preparationCarboxylic acid amides preparationChemical synthesisStructural formula
The invention relates to a method for synthesizing N-(3,4-dimethoxybenzyl)amide capsaicine homologous compounds the general structural formula of which are shown in formula (1), wherein R is alkyl group, naphthenic base or aromatic base the carbon atomic number of which is between 1 and 20. Compared with the existing synthetic method that reagent dimethyl sulfate with toxicity or iodomethane with toxicity and expensive price is used as a methylation regent in the process of synthesizing the compounds, the method of the invention has the maximal advantages that cheap and nontoxic dimethyl carbonate is used as the methylation regent, the reaction conditions are mild and the yield is high.
Owner:ZHEJIANG UNIV
Pharmaceutical composition for therapy of interstitial cystitis
InactiveUS20090105298A1Increase painSymptoms improvedBiocideNervous disorderInterstitial cystitisDepressant
A depressant of capsaicin-sensitive sensory nerve, containing quinuclidin-3′-yl 1-phenyl-1,2,3,4-tetrahydroisoquinoline-2-carboxylate or a salt thereof as an active ingredient, specifically a therapeutic drug of interstitial cystitis, hypersensitive disorder of the lower urinary tract, and / or abacterial prostatitis.
Owner:ASTELLAS PHARMA INC
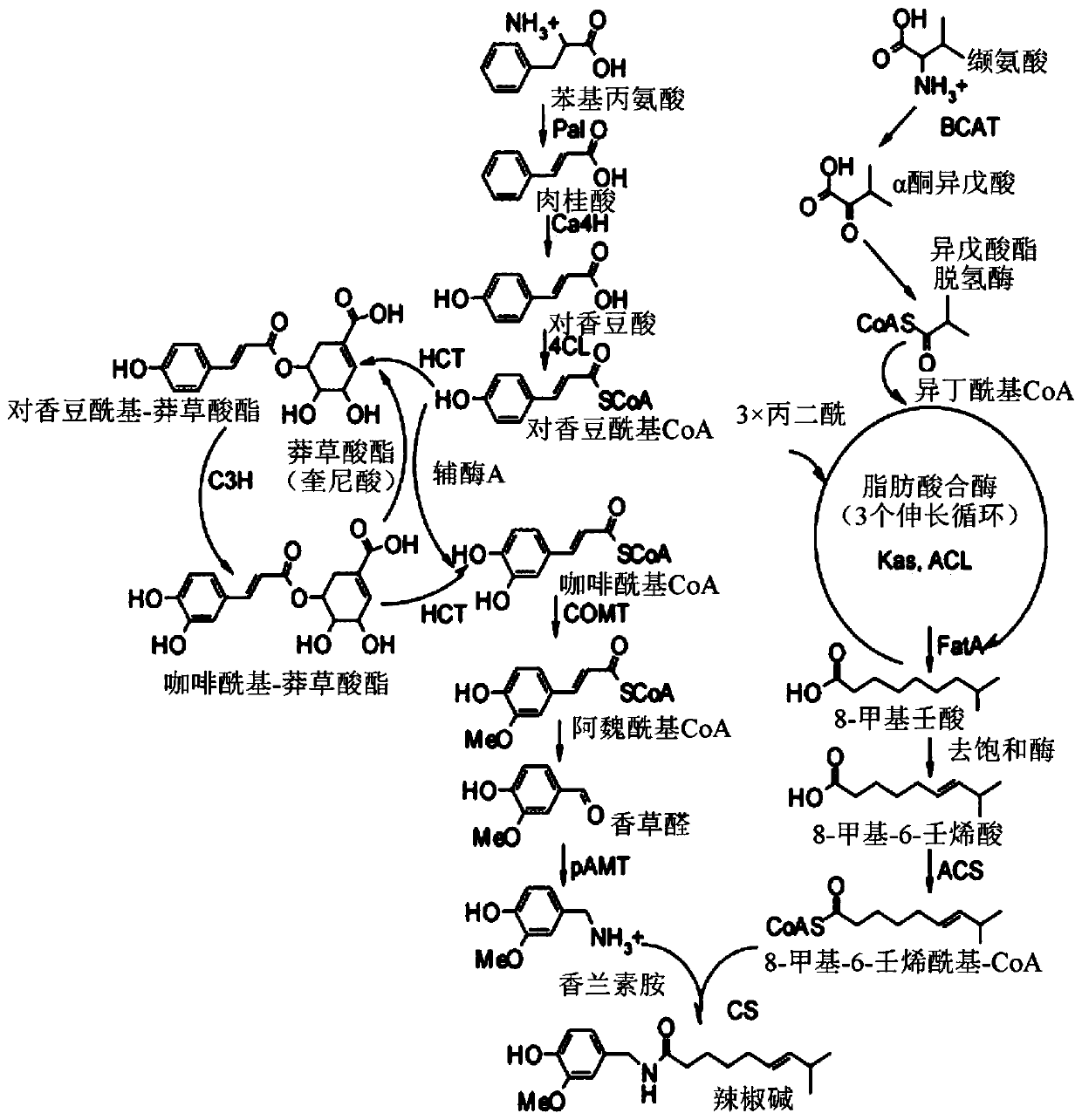
Method for the microbial production of specific natural capsaicinoids
The present invention relates to the production of capsaicinoid compounds including Capsaicin and Nonivamide via microbial fermentation.
Owner:CONAGEN INC
Use of a combination of hypothermia inducing drugs
The present invention relates to the induction of hypothermia in humans in a predictable and dose responsive fashion by use of a pharmaceutical composition comprising a combination of (1) vanilloid receptor agonists, capsaicinoids or capsaicinoid-like agonists reaching and binding to vanilloid receptors, and (2) cannabinoids or cannabimimetic agonists reaching and binding to cannabinoid receptors, thereby inducing hypothermia, thus benefiting patients suffering from illnesses characterized by tissue anoxia.
Owner:NEUROKEY
Antagonists of the transient receptor potential vanilloid 1 and uses thereof
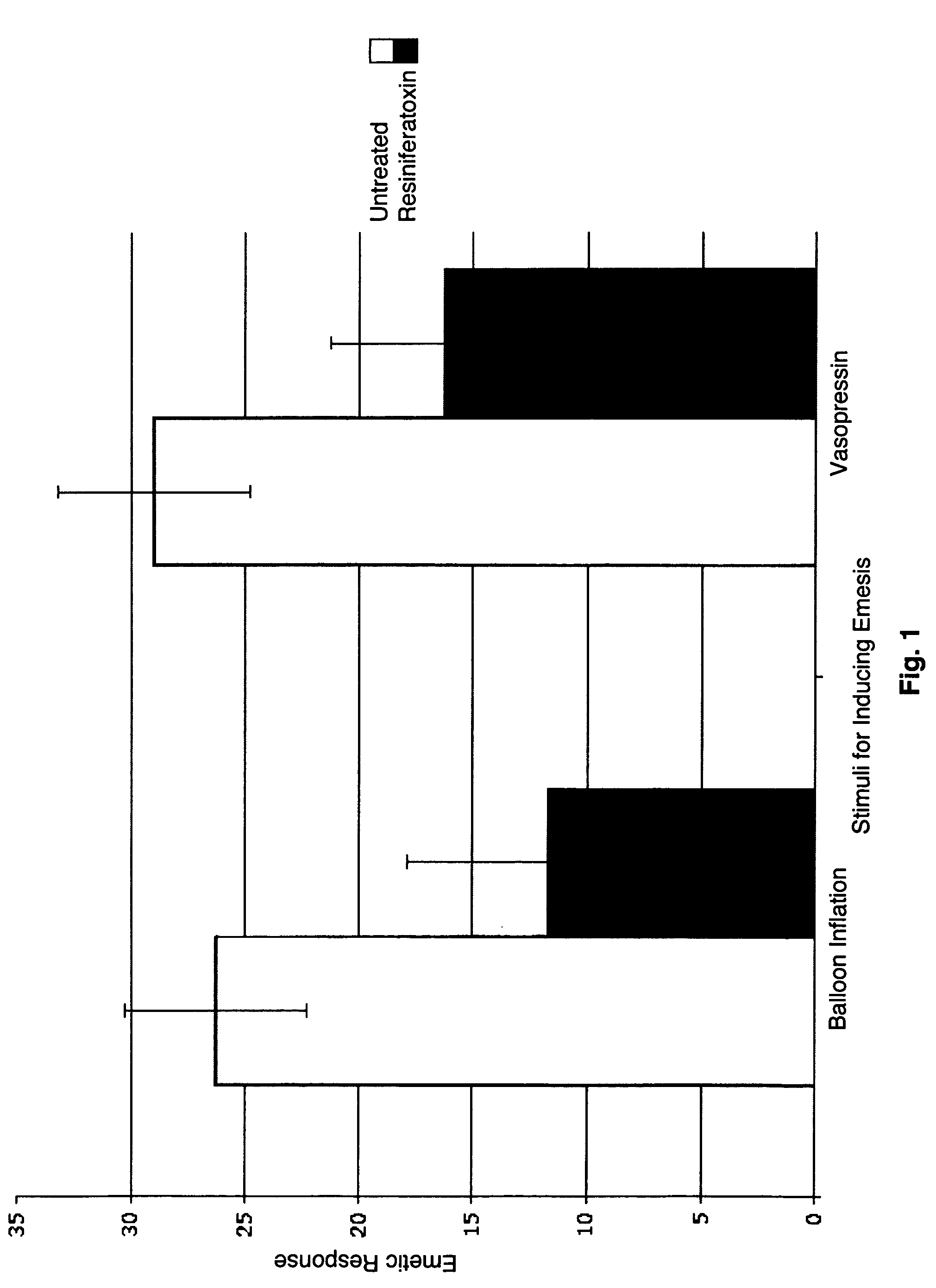
One of the major disabling symptoms of gastroparesis is nausea and vomiting which can be difficult to control with currently available treatments. It is postulated that signaling of gastrointestinal causes of nausea starts with activation of vagal afferent nerves that trigger the central emetic pathway. Most vagal afferent nerves are unmyelinated C-fibers, many of which express the vanilloid receptor TRPV1 and respond to capsaicin. Resiniferatoxin is a very potent capsaicin analogue that has a much more favorable ratio of desensitization to excitation than capsaicin leading to more effective desensitization without irritation. The present invention demonstrates that desensitization of TRPV1 responsive gastric sensory neurons would attenuate nausea and vomiting.
Owner:BOARD OF RGT THE UNIV OF TEXAS SYST
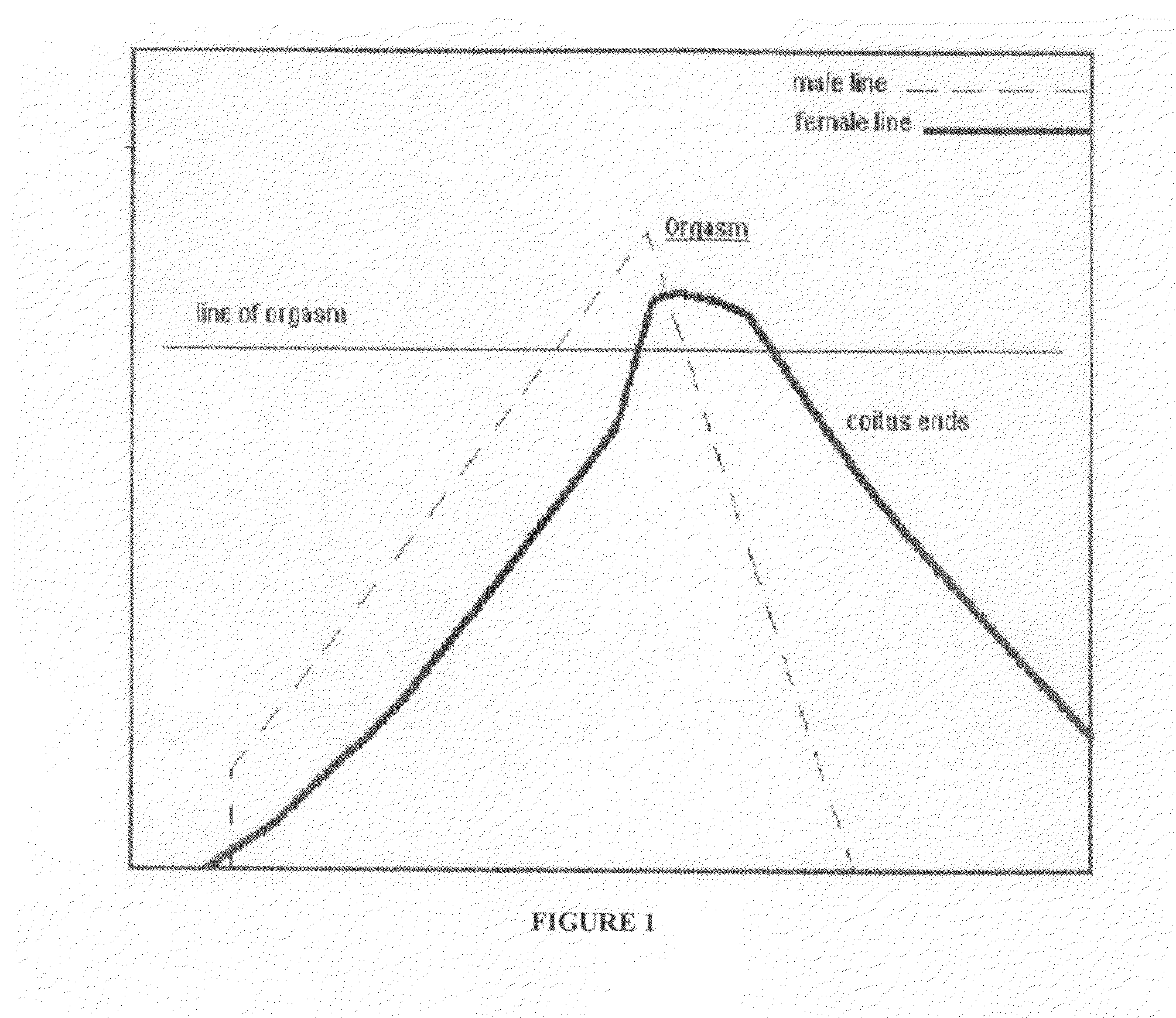
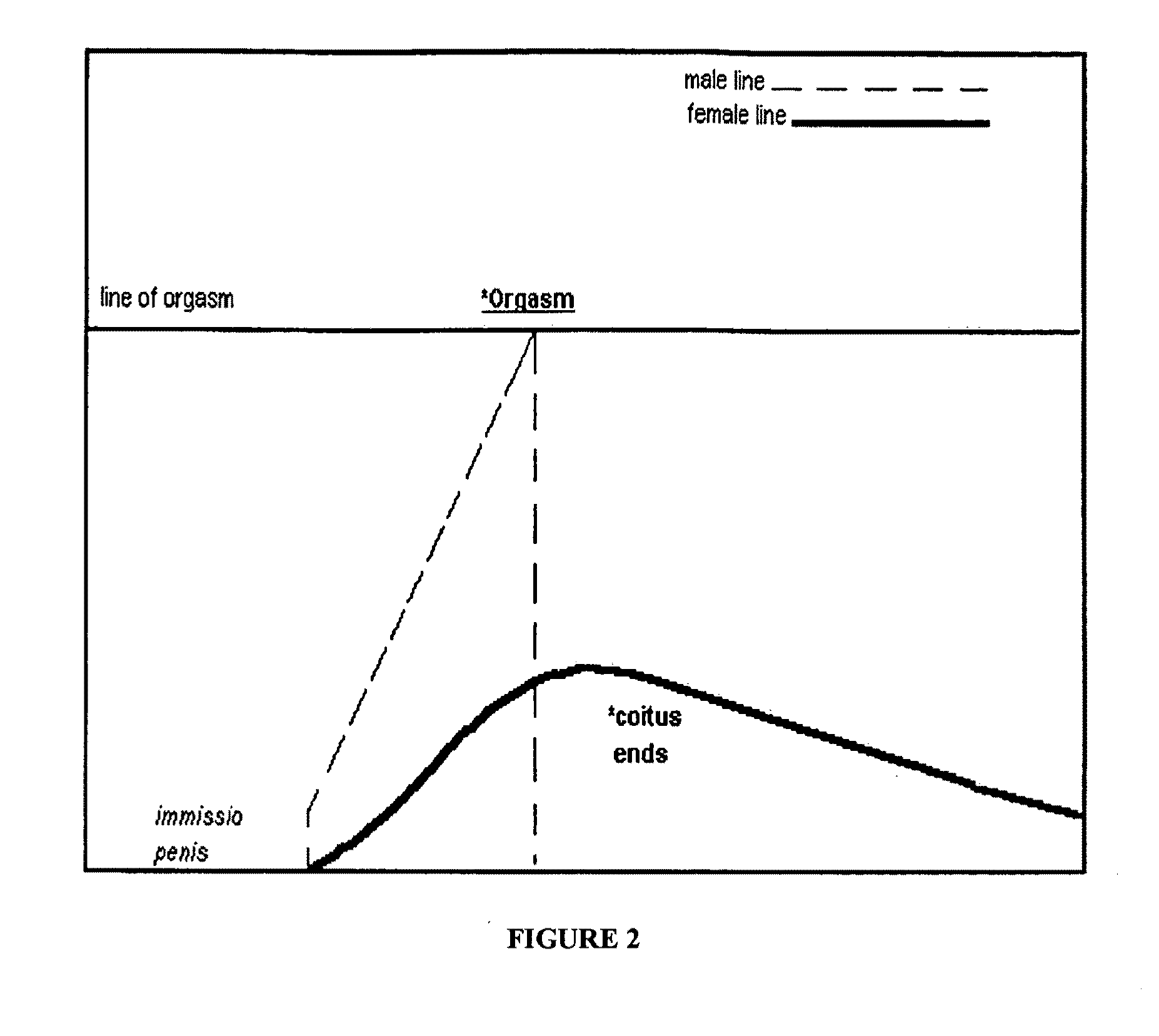
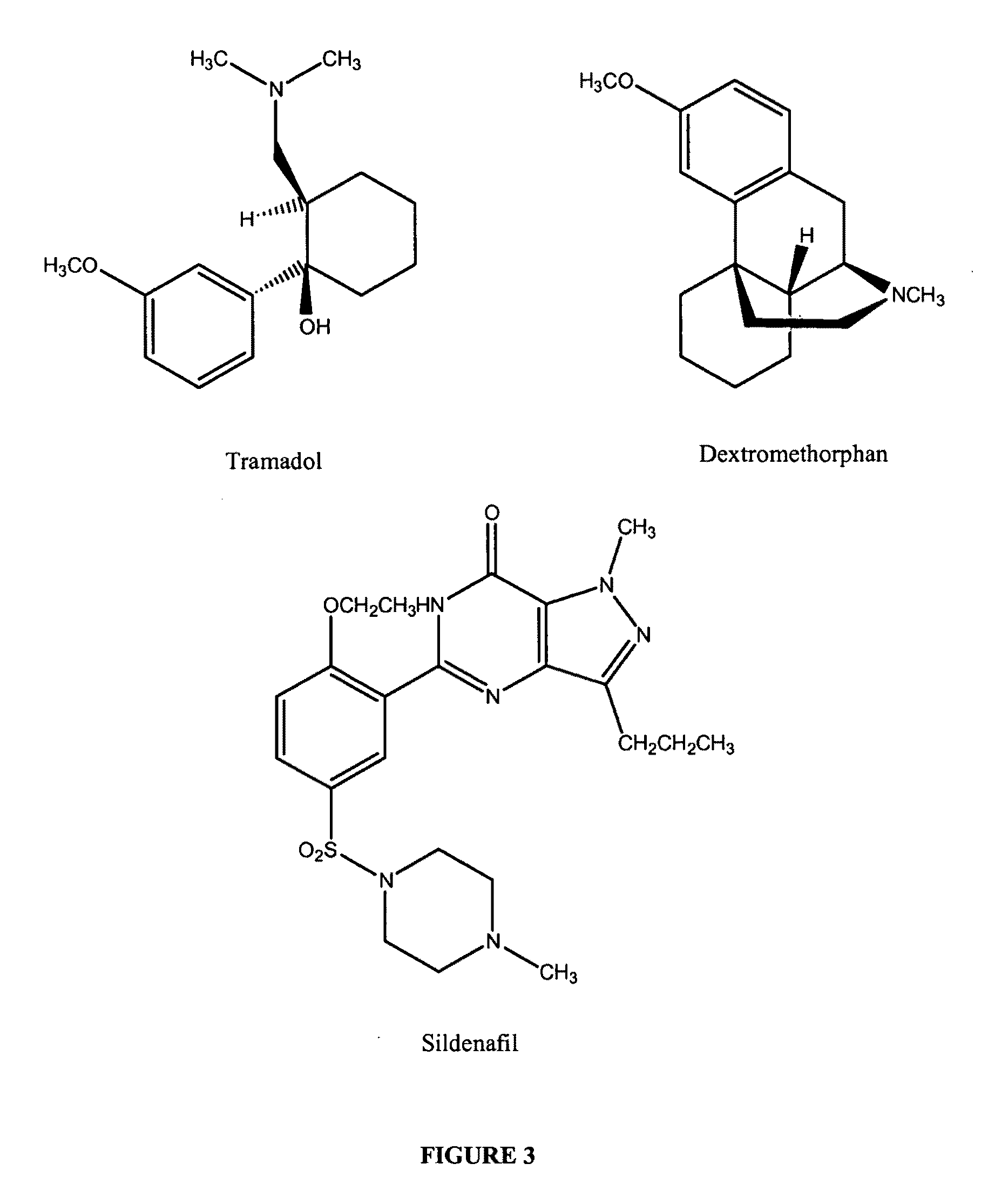
Treatments for premature ejaculation in humans
InactiveUS20100120780A1Reduce abuse potentialOvercome limitationsBiocideSalicyclic acid active ingredientsCITRATE ESTERPhosphodiesterase Type V
Provided are methods and compositions for the treatment of a sexual dysfunction such as premature ejaculation. In certain embodiments, a NMDA antagonist (e.g., dextromethorphan) is administered to a subject in combination with tramadol or a tramadol derivative to treat premature ejaculation. In certain embodiments, a capsaicinoid (e.g., capsaicin) and / or a phosphodiesterase type V inhibitor (e.g., sildenafil citrate) are further administered to the subject. Pharmaceutical preparations such as tablets and capsules are provided.
Owner:TRINITY LAB INC
Preparation method and application of capsaicin-collagen sponge
ActiveCN104490760AImproves transdermal penetrationAvoid first pass effectOrganic active ingredientsPharmaceutical delivery mechanismFreeze-dryingEthylic acid
The invention relates to the technical field of external medicines for hypertrophic scars, and particularly relates to a preparation method and an application of capsaicin-collagen sponge. The preparation method comprises the following steps: 1 preparing collagen powder from tendo calcaneus; 2 extracting the collagen powder with pepsin, so as to obtain an extract liquid; 3 centrifugally collecting supernate; 4 separating out collagen by adding sodium hydroxide, and washing, so as to prepare high-purity collagen; 5 redissolving the collagen with ethylic acid; and 6 evenly mixing the collagen liquid, glycerinum and capsaicin, and then carrying out freeze-drying, so as to obtain the capsaicin-collagen sponge. The capsaicin-collagen sponge prepared by the method is applied to tissue trauma repair and inhibition of the hypertrophic scars in the tissue trauma repair process, and has the advantage of good percutaneous permeability; the drug liver first-pass effect can be avoided; and the thrill to the skin can be reduced.
Owner:GUANGDONG MEDICAL UNIV
Esters of capsaicinoids as dietary supplements
ActiveUS20100120912A1Improve lipophilicityNon-irritation to stomachBiocideNervous disorderSolubilityIrritation
Provided are nutraceutical or dietary supplemental compositions comprising esterified capsaicinoids. The esterified capsaicinoids may converted to the active parent capsaicinoid compound following enzymatic or chemical hydrolysis. In various embodiments, these esterified capsaicinoids have a higher lipophilicity, lipid solubility and result in less irritation to the stomach than the parent capsaicinoid, and hence may be included in certain dietary supplement formulations, including capsules, pills and tablets dietary supplement formulations. The dietary supplement compositions may be used for pain management in mammals in vivo and / or in the treatment of various pathological conditions in humans.
Owner:TRINITY LAB INC
Method for extracting capsaicin crystals from oleoresin capsicum
InactiveCN105418446AReduce extraction timeReduce solvent usageCarboxylic acid amide separation/purificationSolventChemistry
The invention discloses a method for extracting capsaicin crystals from oleoresin capsicum. The method comprises the following steps that chilli powder is extracted with an aqueous alkaline solution in a single-stage continuous countercurrent ultrasonic extraction machine, wherein the aqueous alkaline solution is prepared from sodium chloride, sodium acetate, sodium hydroxide and beta-cyclodextrin; filtrate obtained through filtering separation is processed through the steps of concentration, ethanol extraction, reduced pressure distillation, petroleum ether low-temperature recystalization and the like, and then the high-purity needle-shaped capsaicin crystals are obtained. According to the method, a continuous countercurrent and ultrasonic extraction technique, a micro-cutting interaction technique and the like are combined on the basis of a common solvent method, the extraction time is shortened, the solvent using amount is decreased, the recycle rate and the purity of the capsaicin crystals can be effectively increased, the whole operation process is easy and convenient, the requirements for the reaction condition and production equipment are low, and the method is a capsaicin crystal extraction technology which is suitable for industrialized popularization.
Owner:广西恒得润辣素有限公司
Reverse phase transfer extraction detection process and application of capsaicin compounds in grease
The invention relates to the field of food safety monitoring, and provides a reverse phase transfer extraction detection process and application of capsaicin compounds in grease. The reverse phase transfer extraction detection process comprises the following steps of S1, selecting a proper type of reverse phase transfer extractant, weighing a certain amount of reverse phase transfer extractant, and dissolving the reverse phase transfer extractant in water or buffer salt to prepare a reverse phase transfer extractant solution, S2, mixing the grease with a reverse phase transfer extractant solution according to a certain proportion, S3, fully and uniformly mixing the grease with the extraction solution, S4, directly dripping the mixed solution into a sample adding hole of an immunochromatographic detection card, or directly dipping the mixed solution with immunochromatographic detection test paper, and reacting for a period of time, or standing for 2-3 minutes, separating, taking a lower-layer water phase, and detecting by using an immunochromatography detection card or an enzyme-linked immunosorbent assay (ELISA) method. The method does not need to use an organic solvent, is simpleand convenient to operate and short in detection time, and is suitable for on-site detection or household self-detection of illegal cooking oil.
Owner:UNIV OF SCI & TECH BEIJING
Biocompatible antibacterial film and preparation method thereof
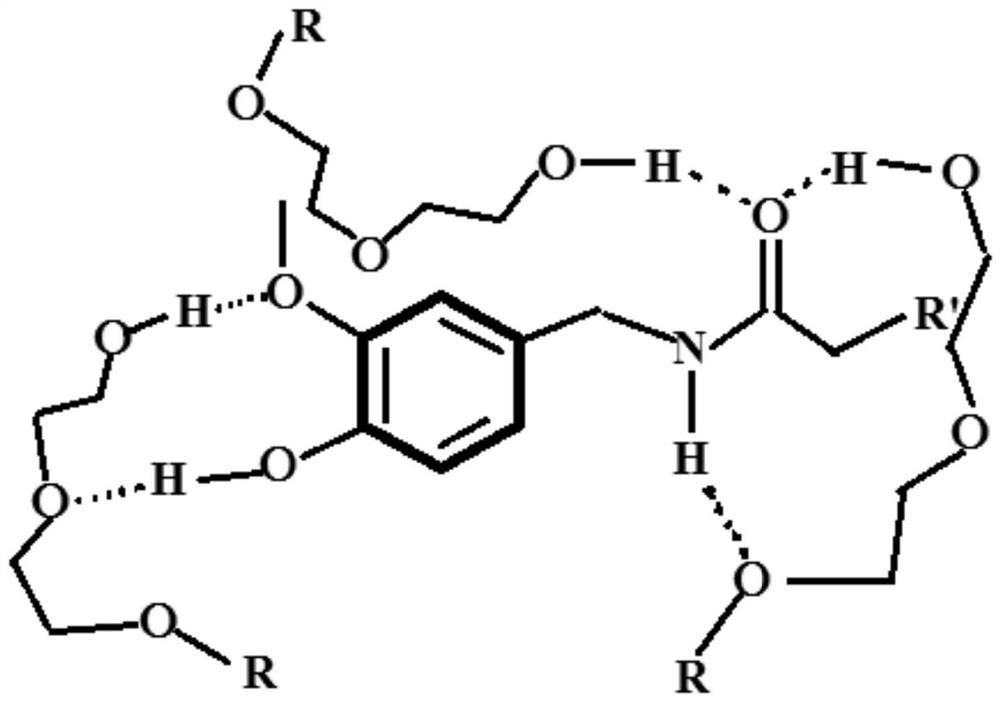
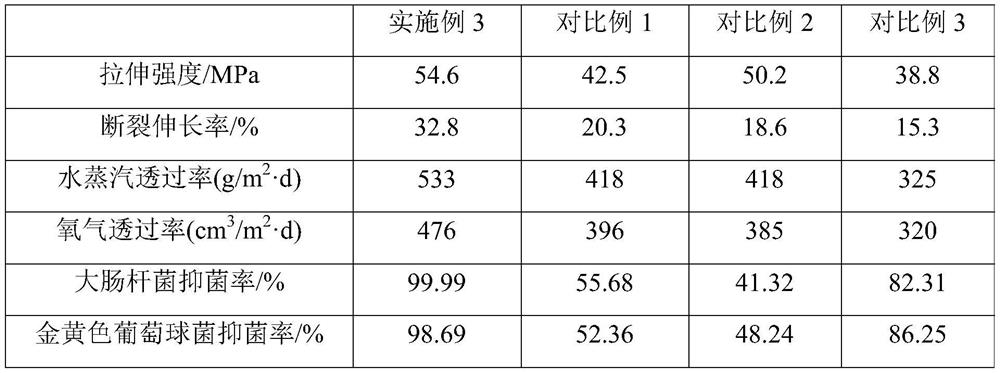
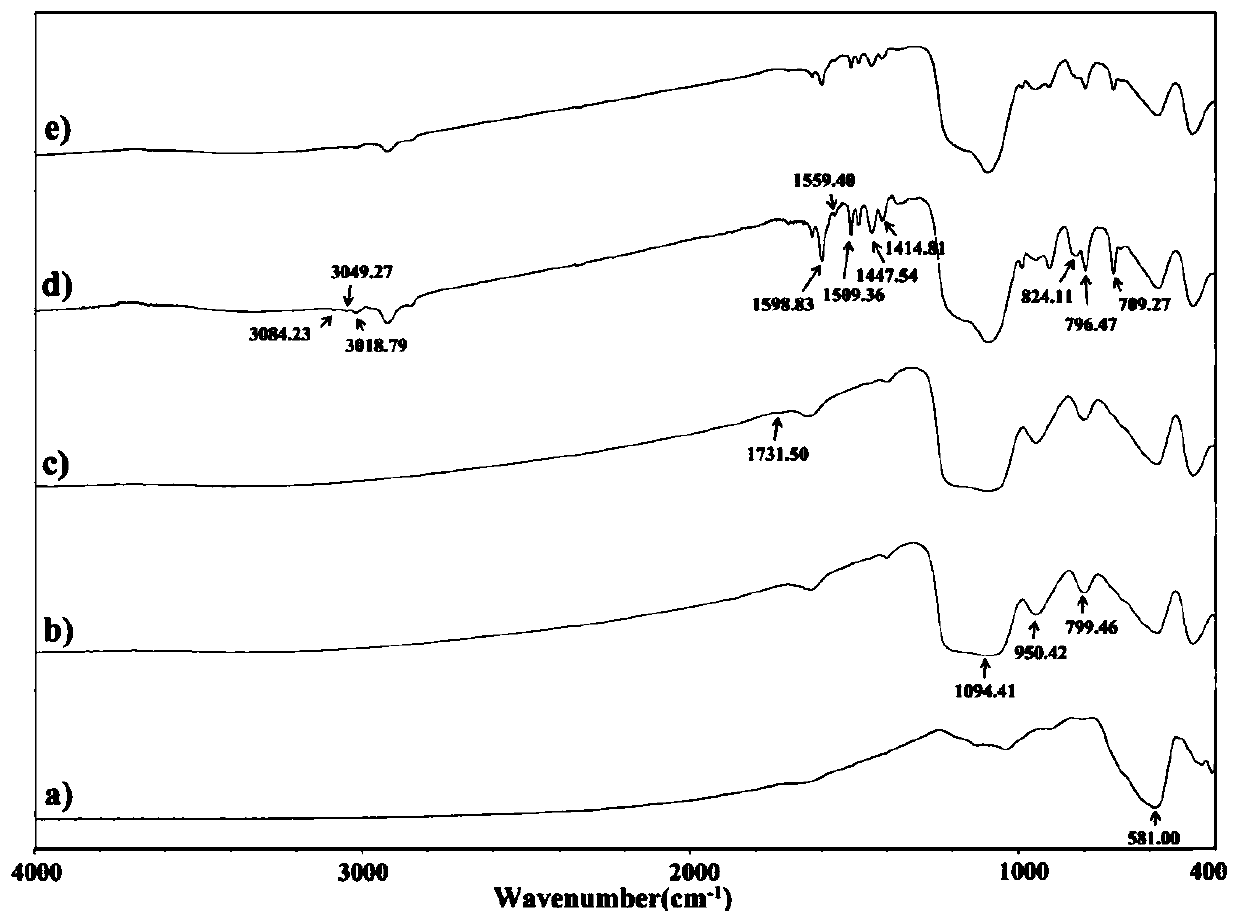
The invention discloses a biocompatible antibacterial film and a preparation method thereof. The biocompatible antibacterial film comprises the following components: a film matrix solution, modified chitosan, capsaicin and glycerol. The film matrix solution is a mixed solution of modified polyvinyl alcohol and starch. According to the biocompatible film disclosed by the invention, polyvinyl alcohol is taken as a matrix polymer and supplemented with starch, chitosan is subjected to modification treatment, a modified chitosan / polyvinyl alcohol / starch composite film is prepared, and capsaicin with antibacterial property is added, so that the film has excellent mechanical property, thermal property, oxidation resistance, barrier property, antibacterial property and biocompatibility.
Owner:ZHEJIANG CHENGDE PACKAGING
Magnetic capsaicin molecularly imprinted polymer and preparation method thereof
ActiveCN110982022AOvercome the disadvantages of difficult elution, large mass transfer resistance, and low binding rateFast recognitionOther chemical processesMass transfer resistanceCapsaicin
The invention discloses a magnetic capsaicin molecularly imprinted polymer and a preparation method thereof. A surface molecular imprinting technology is adopted, magnetic Fe3O4 nanoparticles are usedas a carrier, and molecular imprinting is carried out on the surface of the carrier. The defects of deep recognition sites, difficulty in elution of template molecules, large mass transfer resistance, low binding rate and the like in a traditional method are overcome, the prepared polymer has high recognition speed on the template molecules and certain magnetism, rapid separation can be realizedunder the condition of an external magnetic field, the pretreatment steps are greatly simplified, and the treatment time is shortened.
Owner:CHINA PHARM UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com