Use of telocinobufagin as an analgesic in the treatment of acute and chronic pains
A technology for chronic pain, bufosin, applied in the field of medicine for the treatment or prevention of acute and chronic pain, pain, capable of solving problems such as biological or clinical test results that do not propose inflammation and pain reduction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
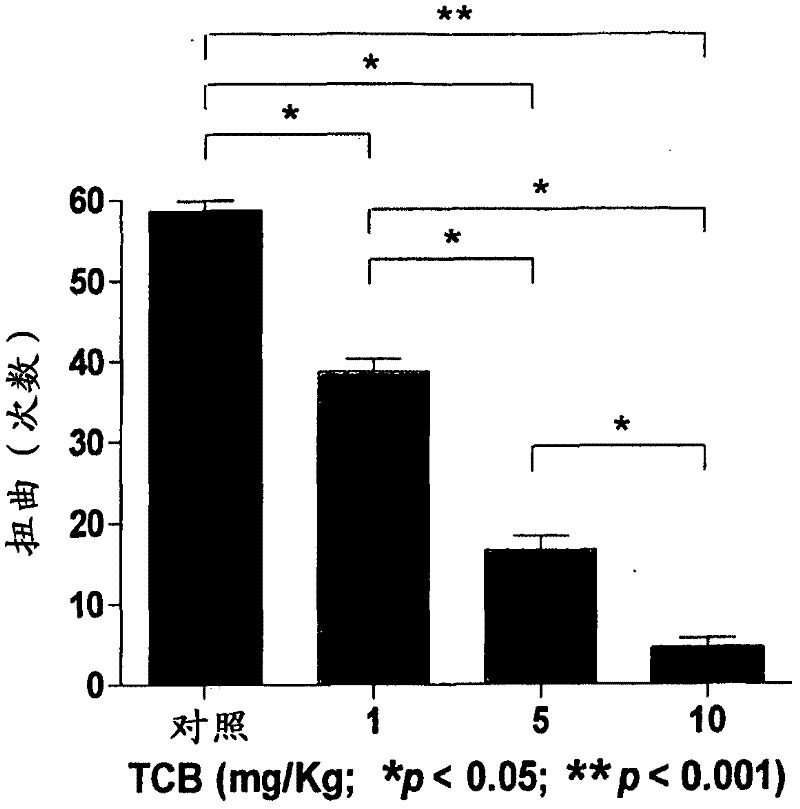
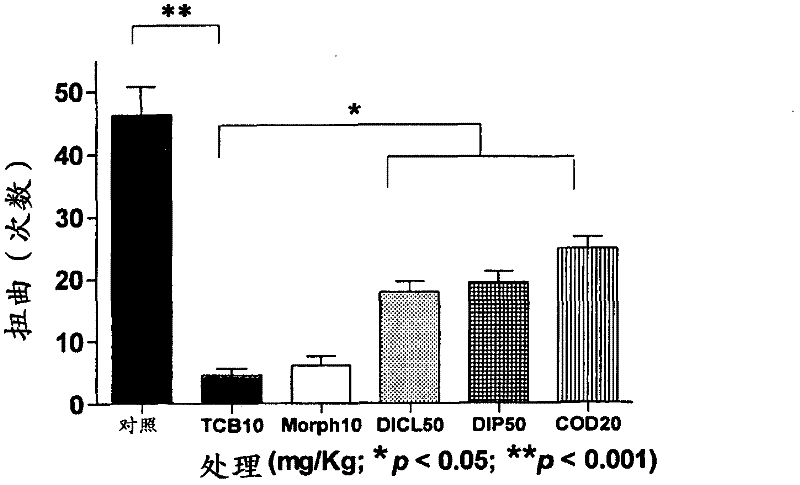
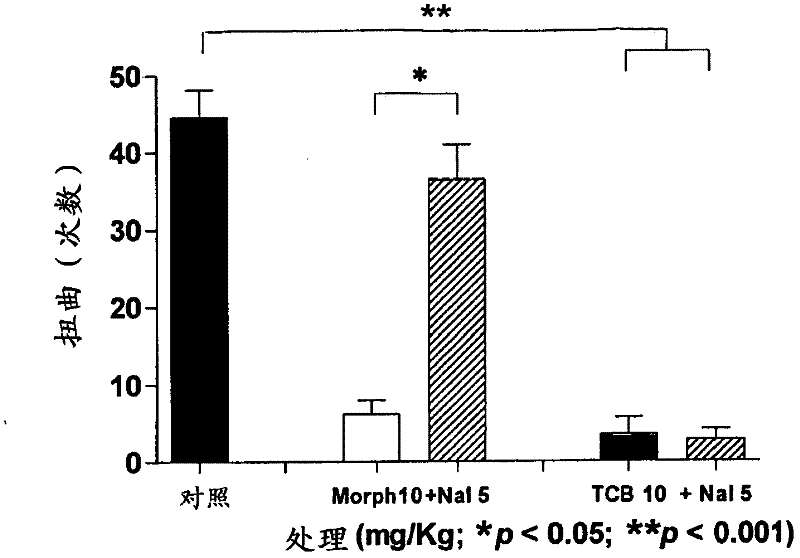
[0105] Example 1 - Torsion Test
[0106] The twist test in mice: distinction between central and peripheral analgesic effects. The test is based on the intraperitoneal administration of a substance that stimulates the serosa, for example, acetic acid, promoting stereotypic behavior in mice, characterized primarily by abdominal contraction, accompanied by stretching of the hind paw. This behavior is considered to be of reflex origin and indicative of visceral pain, similar to symptoms associated with peritonitis. Although the test is not very specific, it is very sensitive and is one of the most commonly used tests for screening new pain relievers. Because contortion tests can assess the analgesic effect of substances that act at both central and peripheral levels, when correlated with specific tests of central action (eg, the hot plate test), it can be demonstrated whether a drug acts at both central and peripheral levels , or only peripheral, or only central. One hour be...
Embodiment 2
[0110] Embodiment 2-formalin test
[0111] Evaluation of neurological and inflammatory acute pain. Intraplantar formalin injections of 4% induced responses of animals to lick or withdraw their paws. The test can evaluate two types of pain: one of neuropathic origin as a result of direct stimulation of nociceptors, a response observed within the first 5 minutes after formalin administration, and the other of inflammatory Origin, characterized by the release of inflammatory mediators, observed within 15 to 30 minutes after formalin injection, expressed as a tonic response to pain. The assay was performed at room temperature (24-25° C.) and in the absence of experimental factors affecting peripheral blood flow that could be caused by greater response sensitivity in the second phase (delayed). One hour before the test, the control group received orally the same volume of vehicle (10 mL / kg) used to dilute the analyzed compound, while the treatment group received increasing dose...
Embodiment 3
[0119] Example 3 - Hot plate test
[0120] Determination of central analgesic effect. The test is to place the animals on a hot plate (52±0.5° C.) and observe how soon they start to show a response, in particular by licking their paws or jumping away. Both responses are considered to be integrated spinal (brain) responses. One hour before the test, the control group received orally the same volume of vehicle (10 mL / kg) used to dilute the analyzed compound, while the treatment group received increasing doses of the test substance under the same conditions. The analgesic effect is determined based on the increase in the wait time for treated animals to lick the paw or jump away compared to the same response of control animals. The results are shown in Figure 10 and 11 middle.
[0121] Figure 10 Dose / response curves for the analgesic effect of oral telocinobufagin (1 mg / kg, 2.5 mg / kg and 5 mg / kg) in the hot plate test are presented. This figure demonstrates that teloci...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com