2,2'-[iminodi(methylene)di-(6-fluoro-3,4-dihydro-2h-1-benzenepyran-2- methanol) methane sulfosalt
A technology of benzopyran and mesylate, which is applied in the field of medicinal chemistry and can solve problems such as harsh conditions, difficulties in mass production, and expensive raw material reagents
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
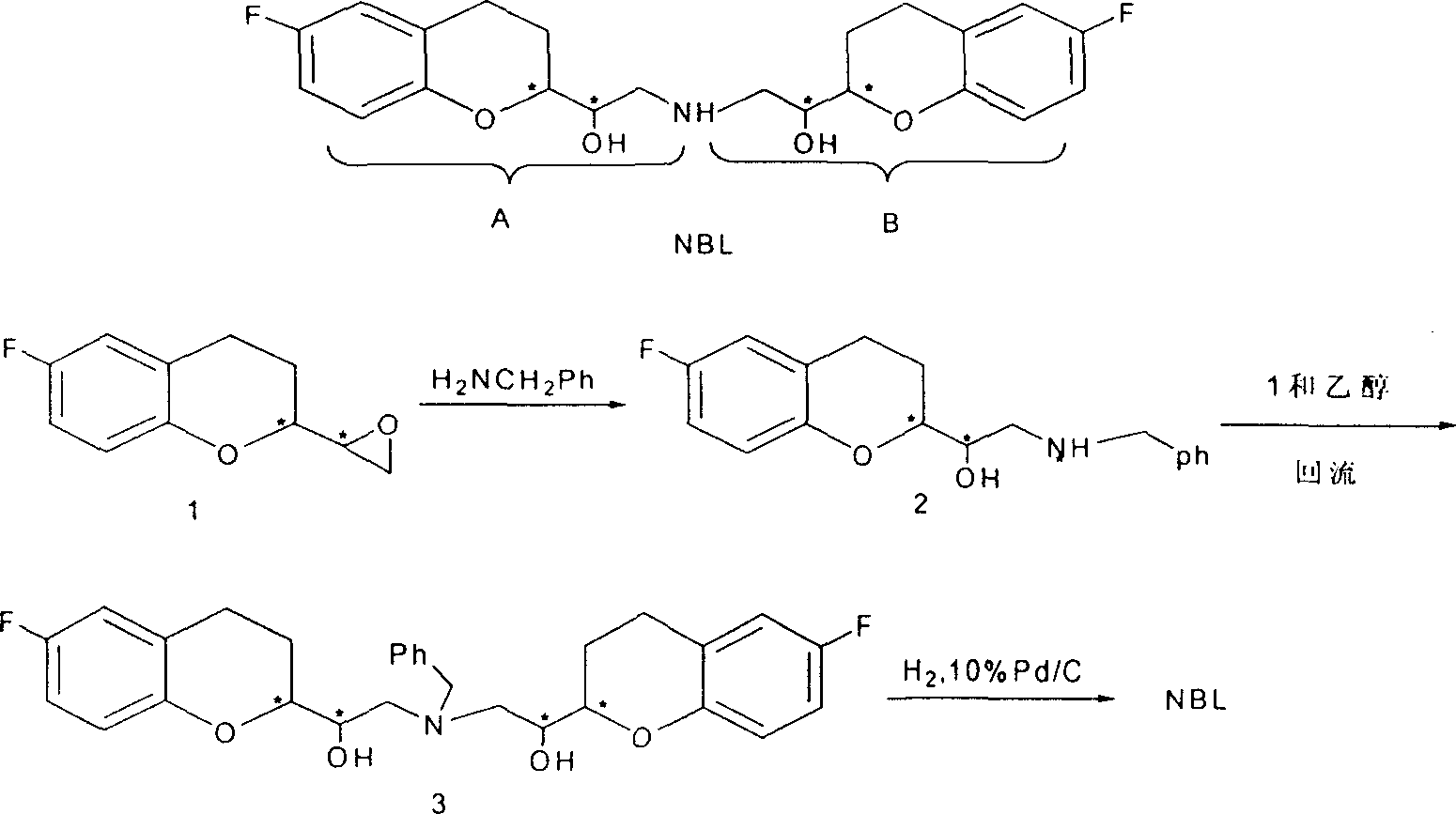
[0028] (1).6-fluoro-3,4-dihydro-2-oxiranyl-2H-1-benzopyran (shown in scheme 2 1) (3.0 grams, 0.016mol), benzylamine (8.3 gram, 0.08mol), isopropanol 100ml, add in the reaction vessel, reflux reaction 2 hours. After stopping the reaction, the solvent was distilled off to obtain a white solid. Recrystallization gave pure white solid 6-fluoro-3,4-dihydro-1-[benzylamino (methylene)]-2H-1-benzopyran-2-methanol] (shown in scheme 2 ) (85% yield), mp 117-118°C.
[0029] (2).6-fluoro-3,4-dihydro-2-oxirane group-2H-1-benzopyran (1 shown in scheme 2) (8.0 grams, 40mmol), the ammoniacal liquor of 15wt% ( 0.27 grams, 4.0mmol), methanol 800ml, after reflux reaction for 2 hours. The solvent was evaporated to obtain a yellow thick substance. Silica gel column purification, using dichloromethane and methanol as eluent. After distilling off the solvent, the white solid 6-fluoro-3,4-dihydro-1-[amino(methylene)]-2H-1-benzopyran-2-methanol (shown in scheme 2) (Yield 78%), mp 119-112°C.
[0...
Embodiment 2
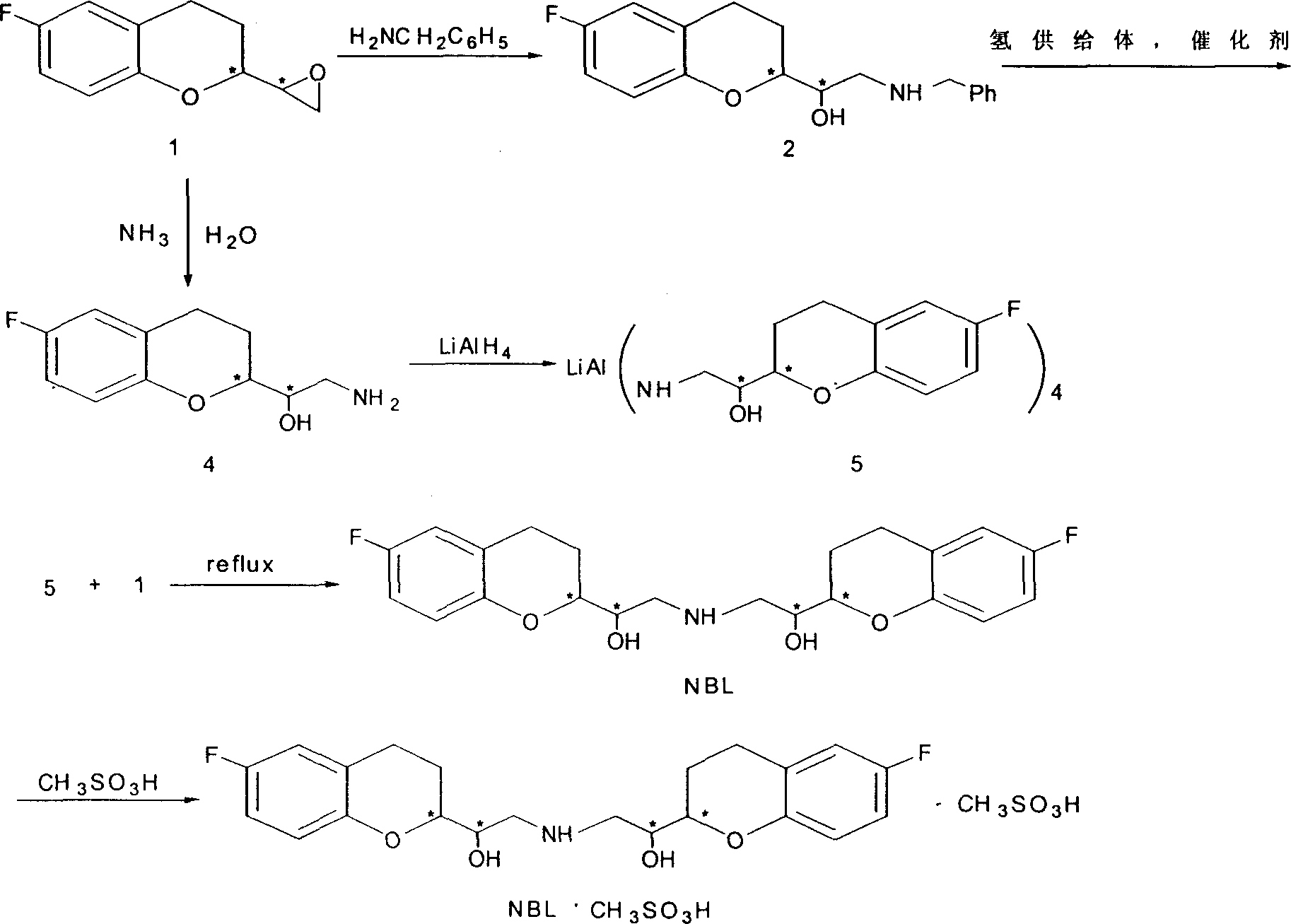
[0035] (1).6-fluoro-3,4-dihydro-2-oxiranyl-2H-1-benzopyran (shown in Scheme 2) (7.5 grams, 0.04mol), benzylamine (0.83 gram, 0.008mol), tetrahydrofuran 20ml, add in the reaction container, reflux reaction 2 hours. After stopping the reaction, the solvent was distilled off to obtain a white solid. Recrystallization gave pure white solid 6-fluoro-3,4-dihydro-1-[benzylamino (methylene)]-2H-1-benzopyran-2-methanol] (shown in scheme 2 ) (85% yield), mp 117-118°C.
[0036] (2).6-Fluoro-3,4-dihydro-2-oxiranyl-2H-1-benzopyran (1 shown in scheme 2) (1.6 g, 8.0 mmol), 25% ammonia water (0.54 g, 8.0 mmol), butanol 70 ml, after reflux reaction for 2 hours. The solvent was evaporated to obtain a yellow thick substance. Silica gel column purification, using dichloromethane and methanol as eluent. After distilling off the solvent, the white solid 6-fluoro-3,4-dihydro-1-[amino(methylene)]-2H-1-benzopyran-2-methanol (shown in scheme 2) (Yield 78%), mp 119-112°C.
[0037] or
[0038] Th...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com