Phthalazinone derivative and preparation method thereof, and catalyst used for preparation of phthalazinone derivative
A derivative, the technology of phthalazinone, which is applied in the field of ionic liquid catalysis, can solve the problems of large amount of ionic liquid catalyst used, complex product purification process, and less cycle times, so as to improve synthesis purity, quality and performance assurance, and improve The effect of utilization
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
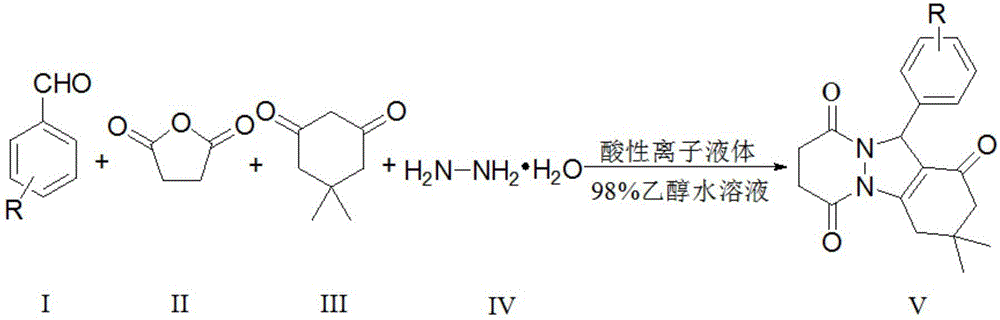
[0034] A kind of preparation method of phthalazinone derivative of the present invention, this method is to be reaction raw material with aromatic aldehyde, succinic anhydride, 5,5-dimethyl-1,3-cyclohexanedione and hydrazine hydrate, in acidic The catalysis of the ionic liquid catalyst prepares the phthalazinone derivative, and its chemical reaction formula is:
[0035]
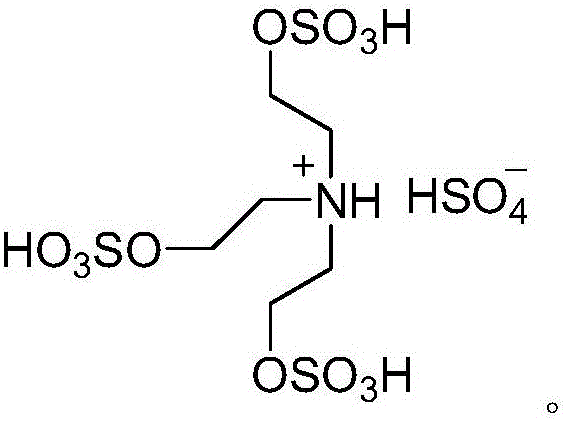
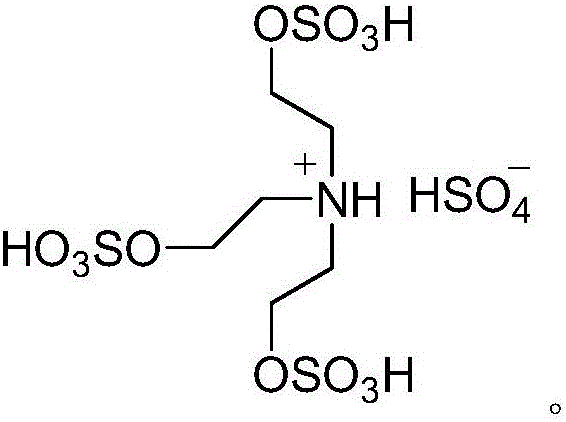
[0036] In the above formula, substance I is aromatic aldehyde, substance II is succinic anhydride, substance III is 5,5-dimethyl-1,3-cyclohexanedione, substance IV is hydrazine hydrate, and substance V is prepared by the present invention 3,4,7,8-tetrahydro-3,3-dimethyl-11-aryl-2H-phthalazin[1,2-α]indole-1,6,9(11H)-trione , the structural formula of the acidic ionic liquid catalyst is:
[0037]
[0038] Aromatic aldehydes in the present invention are benzaldehyde, p-chlorobenzaldehyde, p-nitrobenzaldehyde, m-nitrobenzaldehyde, p-tolualdehyde, p-methoxybenzaldehyde, p-bromobenzaldehyde, 2,4-di Any one of...
Embodiment 1
[0047] Add 1mmol succinic anhydride, 1mmol 5,5-dimethyl-1,3-cyclohexanedione, 1mmol benzaldehyde, 1.2mmol hydrazine hydrate and 0.12mmol acidic ionic liquid to the belt filled with 5ml 98% ethanol aqueous solution In a 50ml single-necked bottle with a stir bar and a condenser. Heat to reflux for 35 minutes, TLC (thin plate chromatography) detection, the raw material point disappears, cool to room temperature, crush the precipitated solid, let stand, filter with suction, wash the filter residue with 98% ethanol aqueous solution, and vacuum dry to obtain 3, 4, 7 , 8-tetrahydro-3,3-dimethyl-11-phenyl-2H-phthalazin[1,2-α]indole-1,6,9(11H)-trione, the yield is 94% , directly add succinic anhydride, 5,5-dimethyl-1,3-cyclohexanedione, benzaldehyde and hydrazine hydrate to the collected filtrate for repeated use.
[0048] 3,4,7,8-tetrahydro-3,3-dimethyl-11-phenyl-2H-phthalazine[1,2-α]indole-1,6,9(11H) obtained in this example - The performance parameters of triketone are as follows:...
Embodiment 2
[0050] Add 1mmol succinic anhydride, 1mmol 5,5-dimethyl-1,3-cyclohexanedione, 1mmol p-chlorobenzaldehyde, 1.1mmol hydrazine hydrate and 0.10mmol acidic ionic liquid to 7ml 98% ethanol aqueous solution in a 50ml single-necked bottle with a stirrer bar and a condenser. Heated to reflux for 28 minutes, TLC (thin plate chromatography) detection, the raw material point disappeared, cooled to room temperature, crushed the precipitated solid, let stand, suction filtered, the filter residue was washed with 98% ethanol aqueous solution, and vacuum dried to obtain 11-(4- Chlorophenyl)-3,4,7,8-tetrahydro-3,3-dimethyl-2H-phthalazin[1,2-α]indole-1,6,9(11H)-trione, The yield is 93%. Succinic anhydride, 5,5-dimethyl-1,3-cyclohexanedione, p-chlorobenzaldehyde and hydrazine hydrate are directly added to the collected filtrate for repeated use.
[0051] The 11-(4-chlorophenyl)-3,4,7,8-tetrahydro-3,3-dimethyl-2H-phthalazine[1,2-α]indole-1,6 obtained in this example , The performance parameters...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com