Use of arachidonic acid for normalization of infradian rhythm
一种花生四烯酸、超昼夜节律的技术,应用在含有效成分的医用配制品、应用、消化系统等方向,能够解决没有昼夜节律同步化功能等问题
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0084] Example 1. Method for producing triglycerides containing arachidonic acid as a constituent fatty acid Law
[0085] Mortierella alpina was used as the arachidonic acid-producing microorganism. Prepared in a 10kL fermenter containing 1.8% glucose, 3.1% defatted soybean meal, 0.1% soybean oil, 0.3% KH 2 PO 4 , 0.1% Na 2 SO 4 , 0.05% CaCl 2 .2H 2 O and 0.05% MgCl 2 .6H 2 O medium (6kL), adjust the initial pH to 6.0. The preculture solution (30L) was inoculated, and the temperature was 26°C, the ventilation was 360m 3 Under the condition of 200kPa per hour and internal pressure, it was placed in ventilated and stirred culture for 8 days. The rate of stirring was adjusted to maintain a dissolved oxygen concentration of 10 to 15 ppm. Regarding the concentration of glucose, the concentration in the medium was set within the range of 1 to 2.5% by the flowing-down method until the 4th day, after which it was maintained at 0.5 to 1% (the % above represents weight (w ...
Embodiment 2
[0088] Example 2. Containing no less than 5% of medium-chain fatty acids linked to 1 and 3 positions and arachidonic acid Preparation of triglycerides with acid linkage to triglyceride (8A8) at position 2
[0089] An ion exchange resin carrier (Dowex Marathon WBA; Dow Chemical; TradeMark) (100 g) was suspended in 80 ml of a 12.5% aqueous solution of Rhizopus delemar lipase (Talipase Powder; Tanabe Seiyaku) and vacuum-dried to produce Fixed lipase.
[0090] Afterwards, 80 g of the triglyceride (TGA 40S) containing 40% by weight of arachidonic acid prepared in Example 1, 160 g of caprylic acid, 12 g of the above-mentioned immobilized lipase and 4.8 ml of water were stirred at 30° C. (130 rpm). React for 48 hours. After the reaction, the reaction solution was removed to produce activated immobilized lipase.
[0091] Then immobilized lipase (Rizopus deliema lipase; Carrier: Dowex MarathonWBA, trade mark) (10g) is packed in the glass column (1.8 * 12.5cm; Volume: 31.8ml) th...
Embodiment 3
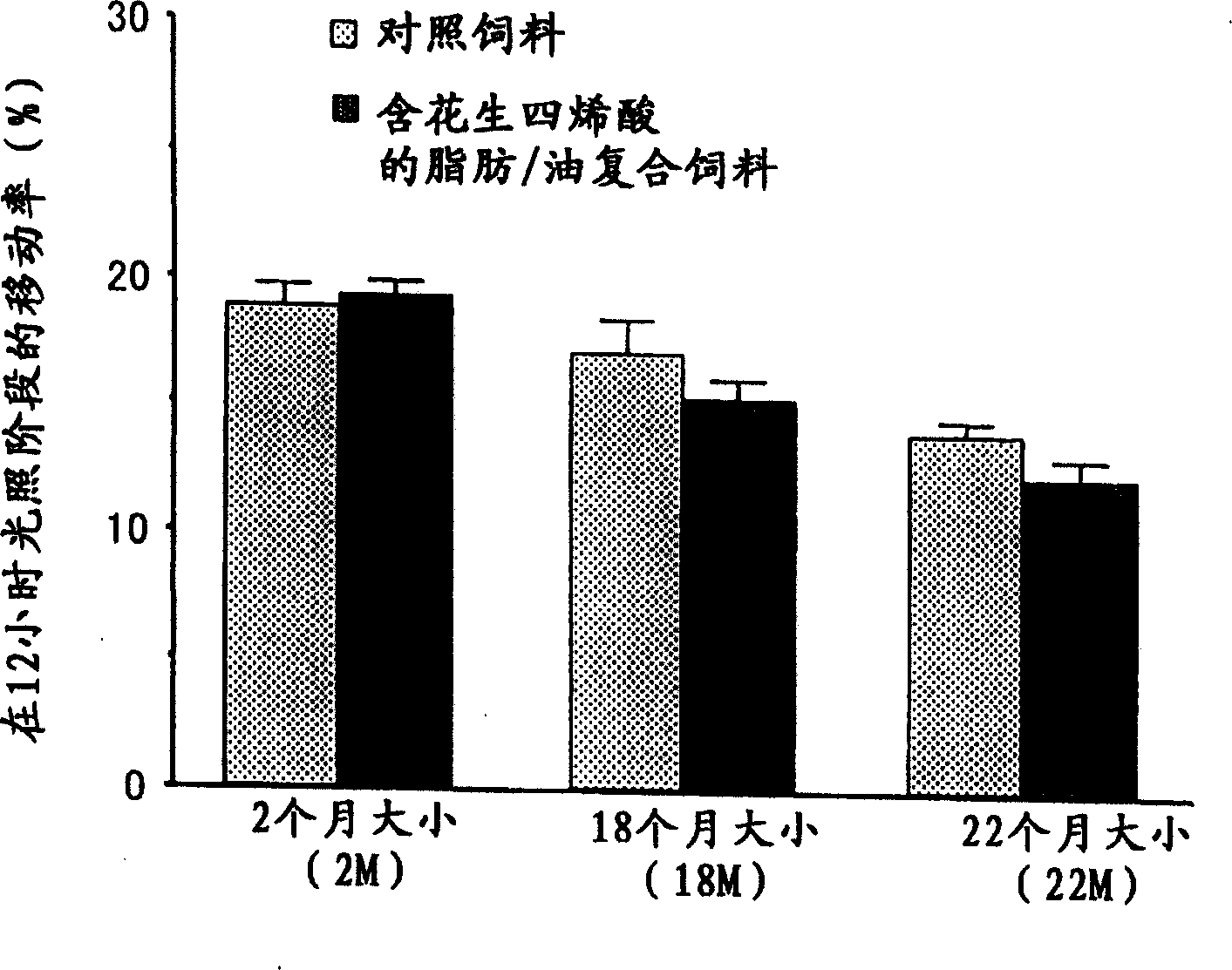
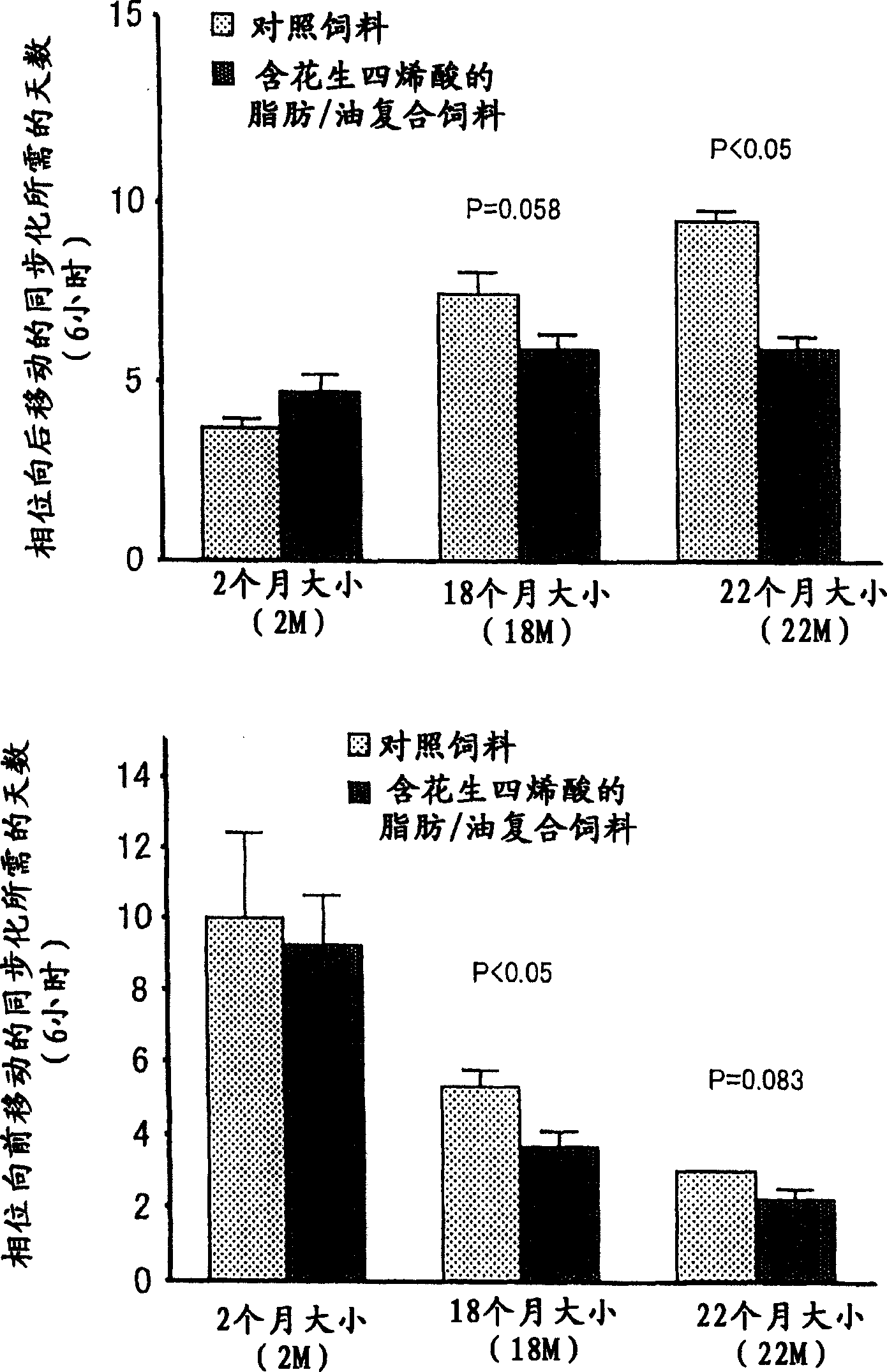
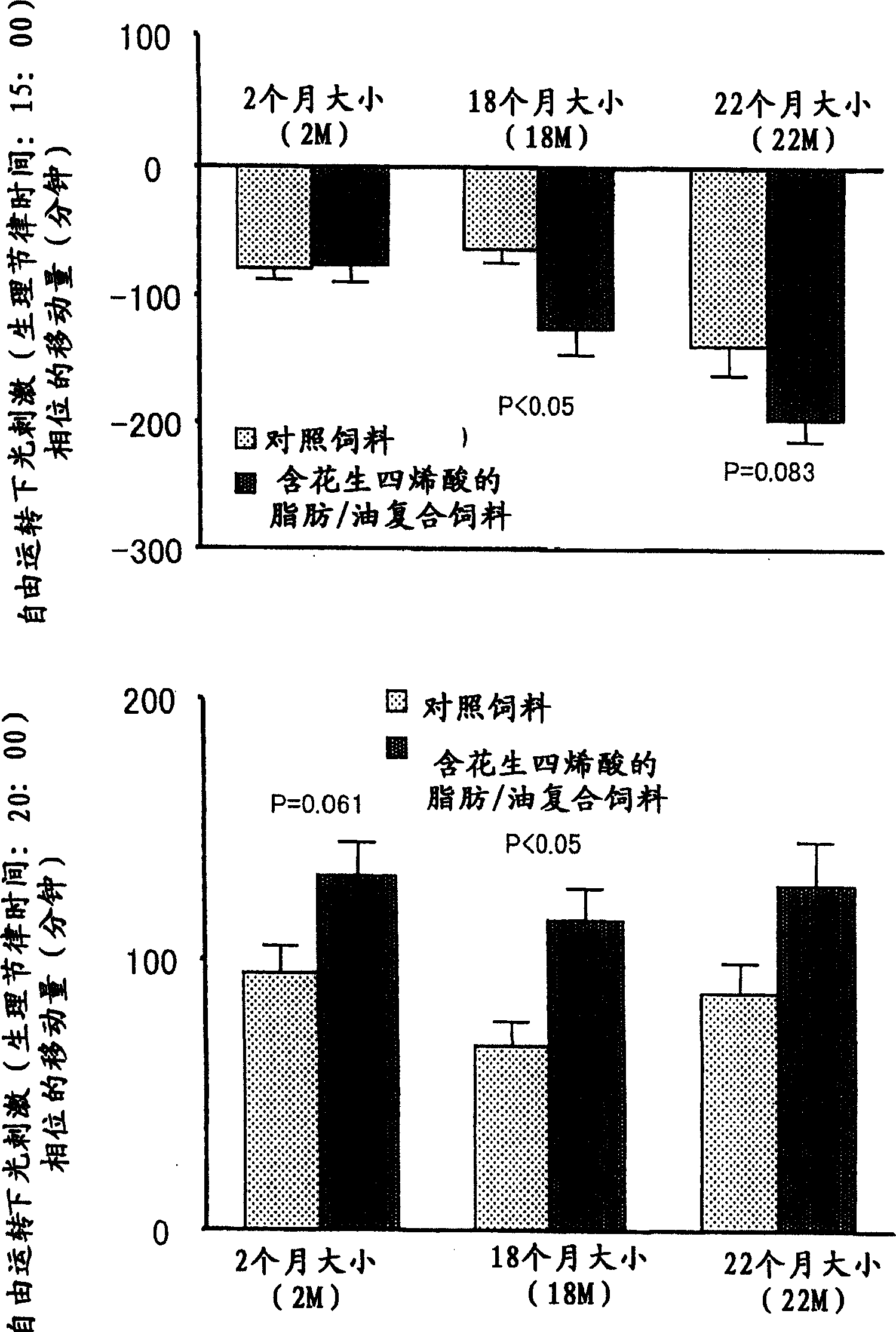
[0093] Example 3. Phase transition of arachidonic acid-containing fats / oils to light and dark cycles (phase change) synchronization promotion
[0094] The effect of the triglyceride (fat / oil containing arachidonic acid) prepared in Example 1 with arachidonic acid as a constituent fatty acid on the phase transition of light and dark cycles was studied in rats.
[0095] Rats have a habit of resting (sleeping) mostly during the light period and active during the dark period. The effect on synchronization was therefore examined on a day-by-day basis by varying the light and dark cycle to its 6 hr preceding and 6 hr post range until synchronization to the new light and dark cycle was achieved. Incidentally, in order to evaluate synchronization, the supracircadian rhythm of rats was studied by continuously measuring the amount of activity during the day and detecting changes in the amount of activity over time during the light period and the dark period.
[0096] Rats were rais...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com