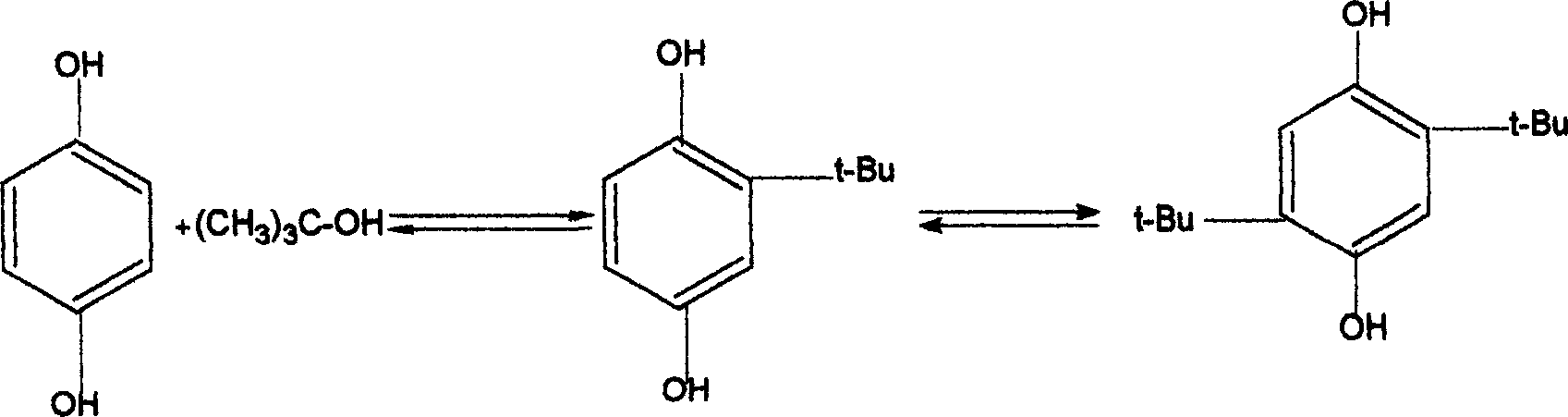
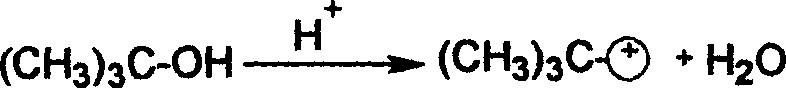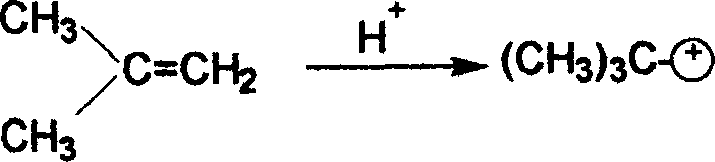2-tertiary-butyl hydroquinone preparation method
A technology of tert-butyl hydroquinone and methyl tert-butyl ether, which is applied in the preparation of high-efficiency polymerization inhibitor 2-tert-butyl hydroquinone, and in the field of high-efficiency antioxidants, can solve the problem of 2-tert-butyl Problems such as low yield of hydroquinone, etc., achieve the effects of easy application and implementation, cost reduction, and simple process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Add 27.5 grams of hydroquinone and 100 ml of toluene into a reactor equipped with a thermometer, a dropping funnel, a stirrer and a reflux condenser, and heat up and stir. When the temperature reached 110° C., the mixed liquid of 20 ml of methyl tert-butyl ether (MTBE) and 2 ml of sulfuric acid was started to be added dropwise. After the dropwise addition is completed, add 11.3ml of methyl tert-butyl ether (MTBE) dropwise. The total addition time should be controlled to be 40min, then continue to stir and react under reflux for 1hr, then add 11.1g of 2,5 tert-butyl p- Hydroquinone (DBHQ), stirred and refluxed for 1 hr, then distilled methanol. The content of 2-tert-butylhydroquinone (TBHQ) in the reaction liquid was 56.1%, and the content of 2,5-tert-butylhydroquinone (DBHQ) was 41.86% through gas chromatography analysis.
Embodiment 2
[0033] Add 27.5 grams of hydroquinone and 81 ml of toluene to a reactor equipped with a thermometer, a dropping funnel, a stirrer and a reflux condenser, heat up and stir. When the temperature reaches 85°C, start to drop the mixed liquid of 33.3ml of methyl tert-butyl ether (MTBE) and 2.66ml of sulfuric acid, and finish the dropwise addition within 20min. After stirring and reacting under reflux for 1 hr, 13.9 g of 2,5 tert-butylhydroquinone (DBHQ) was added, and reflux was continued for 1 hr, and methanol was distilled off. The content of 2-tert-butylhydroquinone (TBHQ) in the reaction solution was 57.97%, and the content of 2,5-tert-butylhydroquinone (DBHQ) was 38.96%.
Embodiment 3
[0035] Add 27.5 grams of hydroquinone and 135 ml of toluene into a reactor equipped with a thermometer, a dropping funnel, a stirrer and a reflux condenser, and heat up and stir. When the temperature reached 105°C, 33.3ml of methyl tert-butyl ether (MTBE) and 2.66ml of sulfuric acid mixed liquid were added dropwise, and the addition was completed within 60 minutes. After stirring and reacting for 45 minutes under reflux, 11.1 g of 2,5 tert-butylhydroquinone (DBHQ) was added, and the reaction was continued under reflux for 45 minutes, and then methanol was distilled off. The content of 2-tert-butylhydroquinone (TBHQ) in the reaction solution was 60.01%, and the content of 2,5-tert-butylhydroquinone (DBHQ) was 36.98%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com