Cladribine formulations for improved oral and transmucosal delivery
A technology of transmucosal and oral agent, applied in the field of compositions containing cladribine-cyclodextrin complex, can solve the problems of high cost, low bioavailability and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
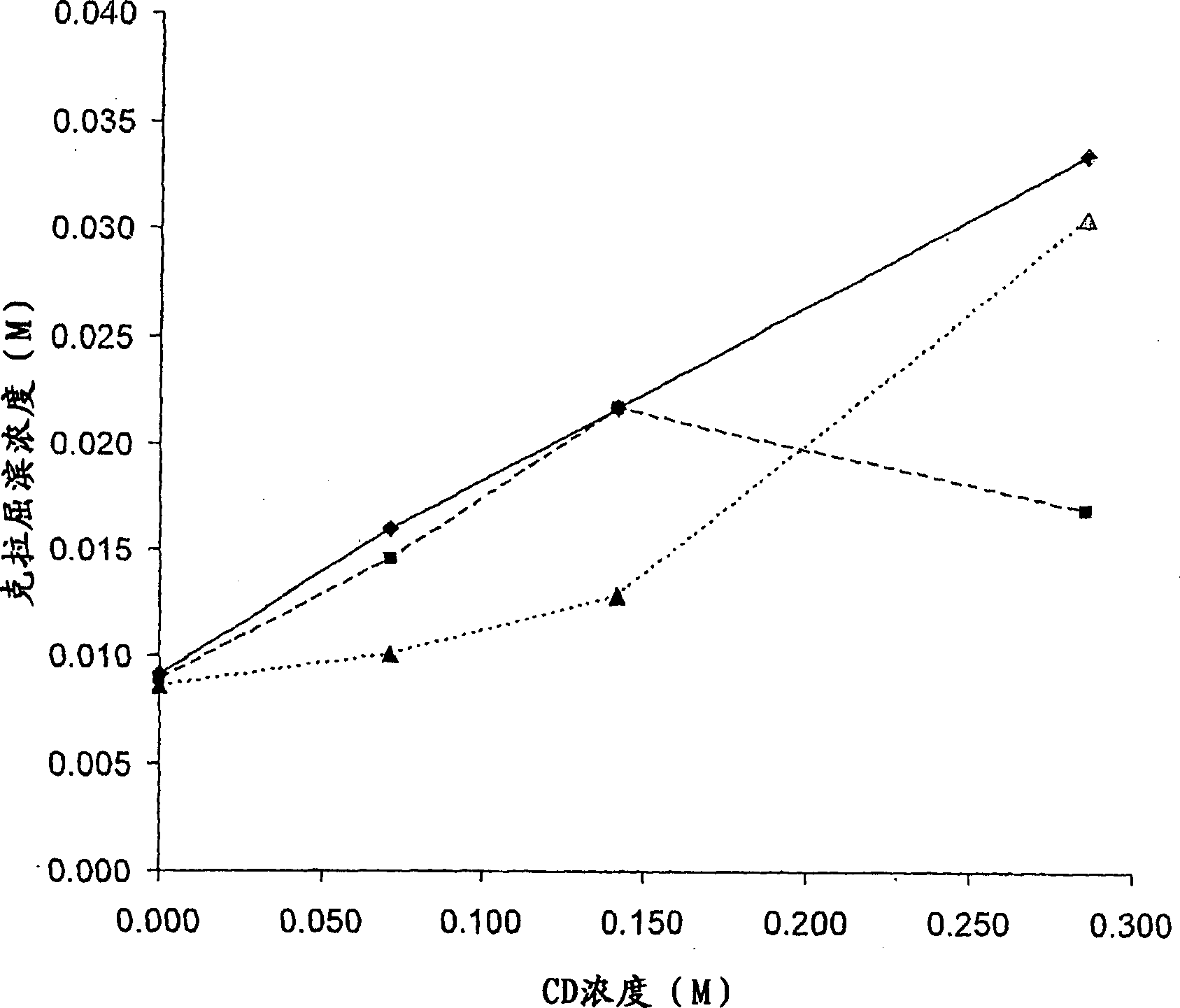
[0076] Embodiment 1 phase solubility research
[0077] Phase solubility studies were performed as follows. Excess cladribine was added to cyclodextrin solutions of various concentrations of γ-cyclodextrin (γCD) or hydroxypropyl-β-cyclodextrin (HPβCD) and complexed as described in Example 2 below. Additionally, in one set of experiments, the effect of hydroxypropylmethylcellulose (HPMC) on compounding was investigated. Remove excess undissolved cladribine by filtration. Data points were obtained by detecting the amount of cladribine in the complex solution. The method was repeated using different known concentrations of cyclodextrin until several data points were obtained. These data points are then plotted, each data point representing the maximum amount of cladribine that can be complexed with a particular concentration of cyclodextrin, ie each point represents a saturated cladribine-cyclodextrin complex. Points on the line generated from the data points represent HTA rat...
Embodiment 2
[0083] The preparation of embodiment 2 cladribine-cyclodextrin complex
[0084]Part A: Cladribine was complexed with HPβCD or γCD by the following general method.
[0085] An excess aqueous suspension of cladribine and a concentrated solution of cyclodextrin (about 27% for γ-cyclodextrin; about 40% for HPβCD) were mixed with stirring at room temperature for about 9 hours. This will strike a balance. Excess uncomplexed cladribine that may be present is removed by filtration. To form a solid saturated cladribine-cyclodextrin complex, the aqueous cladribine-cyclodextrin solution was lyophilized prior to incorporation into solid buccal or oral tablets. The freeze-drying method comprises: the complex solution is rapidly dropped to a temperature of about -40°C to about -80°C for about 2 to 4 hours, preferably about 3 to 4 hours of freezing stage, for example, the temperature of about -45°C is maintained for about 200 minutes , followed by a primary drying stage at about -25°C for...
Embodiment 3
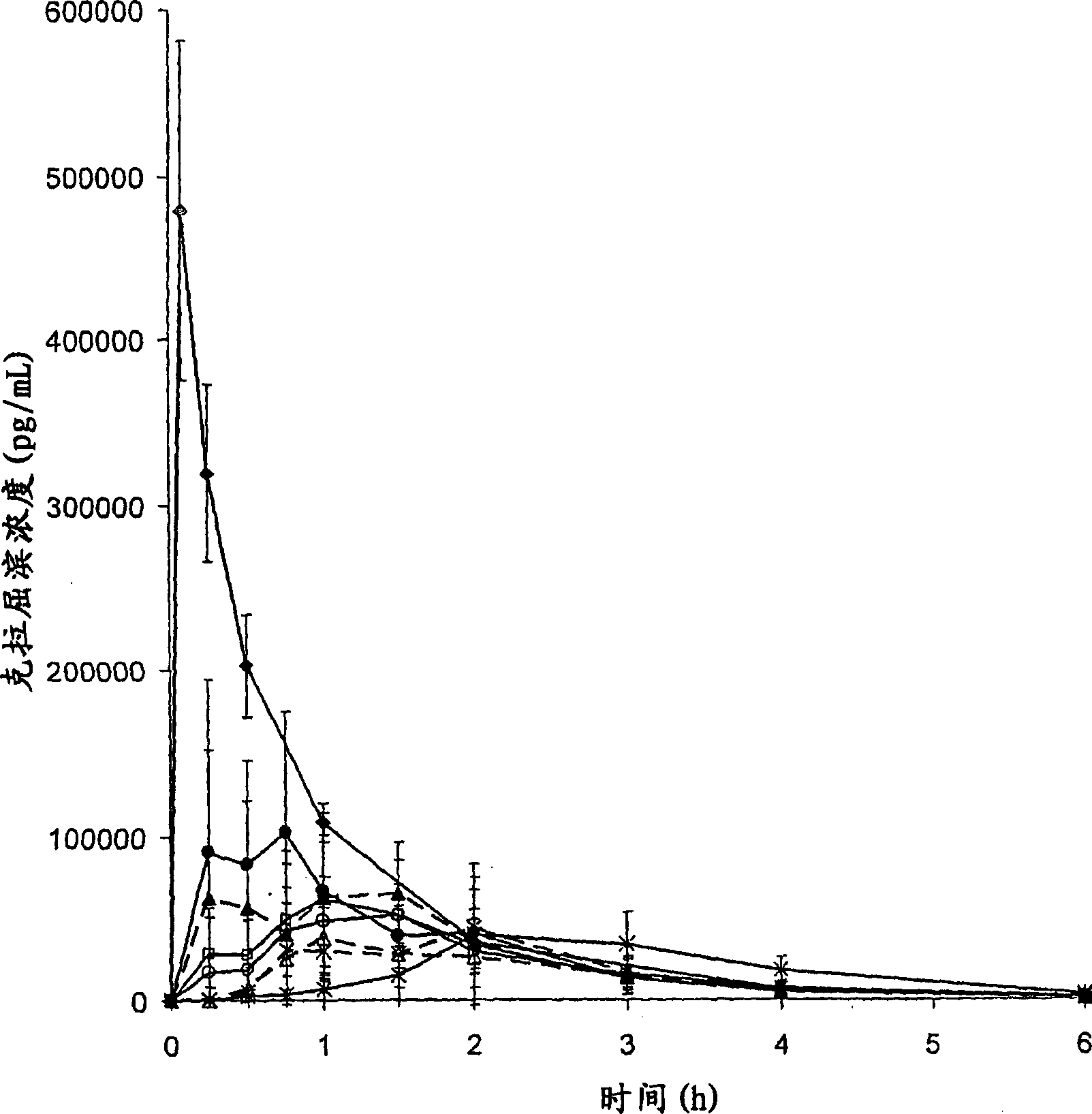
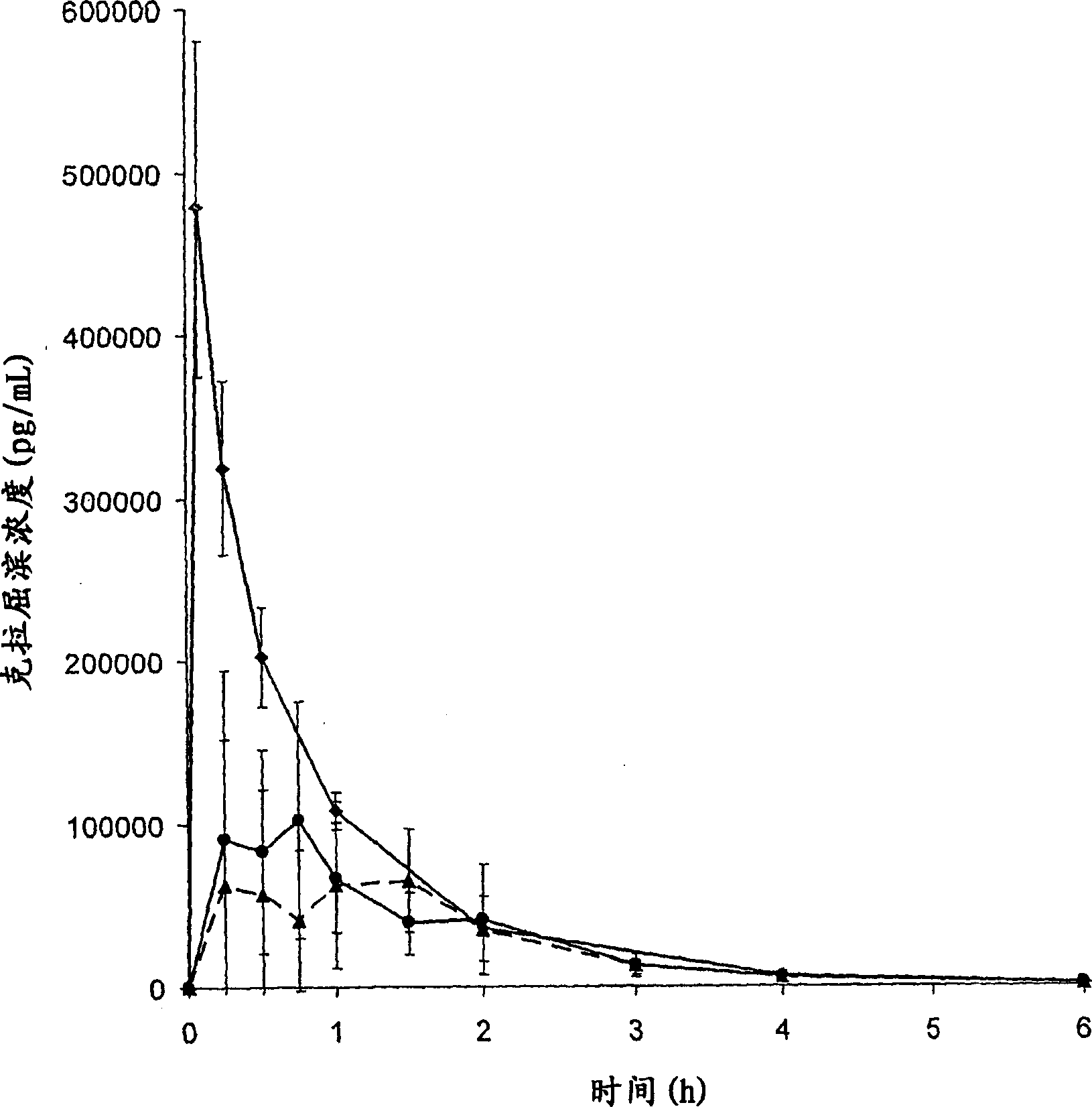
[0095] Pharmacokinetic Studies
[0096] The bioavailability of cladribine when complexed with γCD or HPβCD was evaluated in the beagle retriever model. Data from this model are expected to be representative for human applications.
[0097] Saturated cladribine-cyclodextrin complexes FD02 and FD03 prepared as in Example 2, part B, were used for the preparation of oral and buccal tablets. The composite material and magnesium stearate were passed through a 18 mesh (0.9 mm) screen, mixed for 5 minutes, and compressed using a 10 mm punch. The 10 mm tablet has a slightly convex upper shape and a flat beveled lower shape. The production formula is as follows:
[0098] batch number
RDT-0418 / C
RDT-0418 / D
Element
mg / tablet
mg / tablet
cladribine / γ-CD complex
FD02
232.65 *
cladribine / 2-HPβCD complex
FD03
212.85 *
2.35
2.15
Total
235.00
2...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com